Composite for treating cancer containing oligonucleotide and nontoxic LPS
A technology of composition and deoxynucleotide, which can be used in medical preparations containing active ingredients, drug combinations, organic active ingredients, etc., and can solve problems such as sepsis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: screening avirulent strain
[0037] Screening and discovery of Escherichia coli mutants with very short lipopolysaccharide
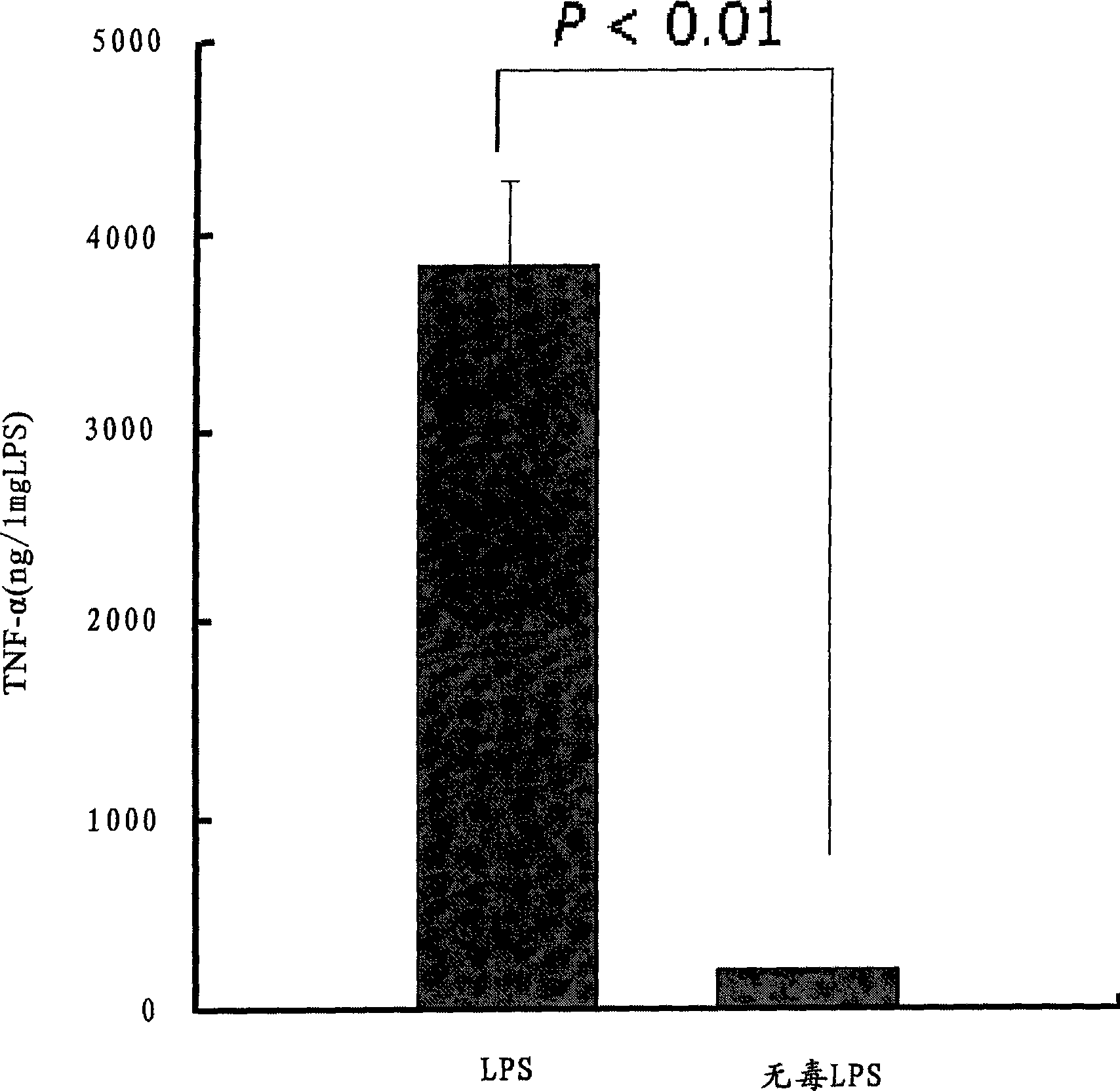
[0038] The Escherichia coli (E.coli) strain EG0021 having a very short lipopolysaccharide sugar chain was discovered from Escherichia coli living in the intestinal tract of healthy people, and the Escherichia coli (E.coli) strain EG0021 was established Method for purifying lipopolysaccharide.
[0039] A single colony of Escherichia coli (E. coli) obtained from a healthy male adult was cultured in a liquid medium, followed by repeating the selection operation 5 times to obtain 50 strains of Escherichia coli (E. coli). And, get each bacterium colony from the 50 strains selected on the plate, dissolve in 4ml of 0.9% physiological saline, then take 1ml of the resulting solution and transfer it to an Eppendorf centrifuge tube (Eppendorf tube), and use 2 μl DNase I treatment was performed, followed by reaction at 37° C. for 1 hour in...
Embodiment 2
[0041] Example 2: CG Methylation
[0042] To characterize the function of unmethylated CGs in oligonucleotides, cytosine residues of CG sequences were selectively methylated using SssI methylase.
[0043] DNA methylation was performed by mixing 1 unit of CpG methylase (M.Sss I; NEB M0226S) with 10 µg of ODN7909, followed by mutual reaction at 37°C for 12 hours. At this time, 160 µ of MS-adenosylmethionine (SAM) as a methyl donor was mixed and reacted together.
[0044] After complete methylation, residual salts and enzymes were then removed using DNA cleanup kit (CPG DPC60050) and Micropure-EZ (Amicon 42529).
Embodiment 3
[0045] Example 3: Purification of CIA02 from mutant Escherichia coli (E.coli)
[0046] Purification of lipopolysaccharide from mutant Escherichia coli (E.coli)
[0047] Escherichia coli (E. coli) strains were prepared in the same manner as in DNA isolation.
[0048] The strain was then mixed with 2 volumes of ethanol, centrifuged at 4,000 g to settle the pellet, and then 1.5 volumes of acetone was added to the resulting pellet, thoroughly mixed and centrifuged at 4,000 g.
[0049] An equal amount of diethyl ether was added to the resulting precipitate, mixed thoroughly and centrifuged at 4,000 g. The cell pellet obtained by centrifugation was covered with perforated aluminum foil, dried, and the cell body was weighed, followed by adding an extraction mixture (90% phenol:chloroform:petroleum ether=2:5: 8).
[0050] The resulting mixture was divided into glass centrifuge tubes, and centrifuged at 25° C. and 3,000 rpm (1,200 g) for 20 minutes to obtain a supernatant. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com