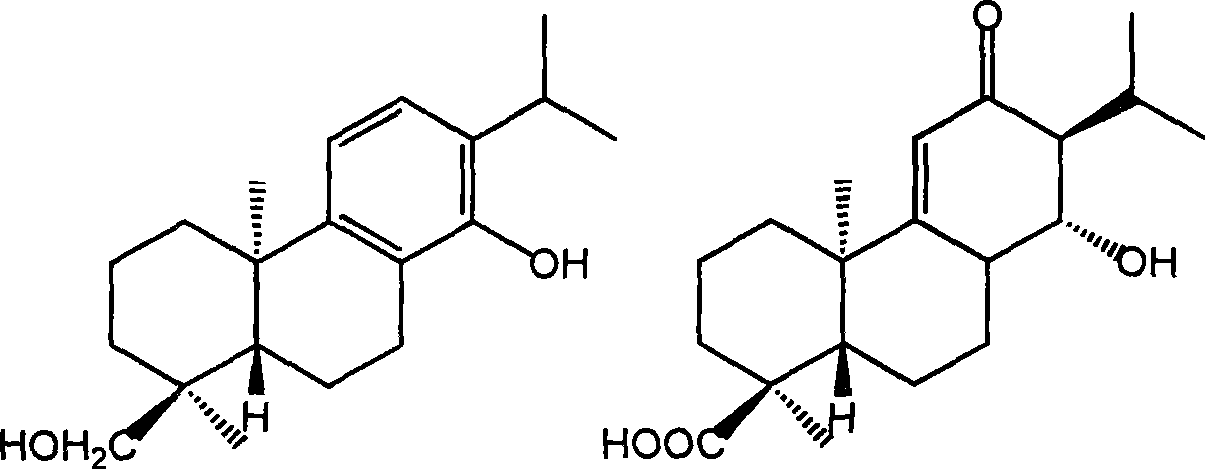
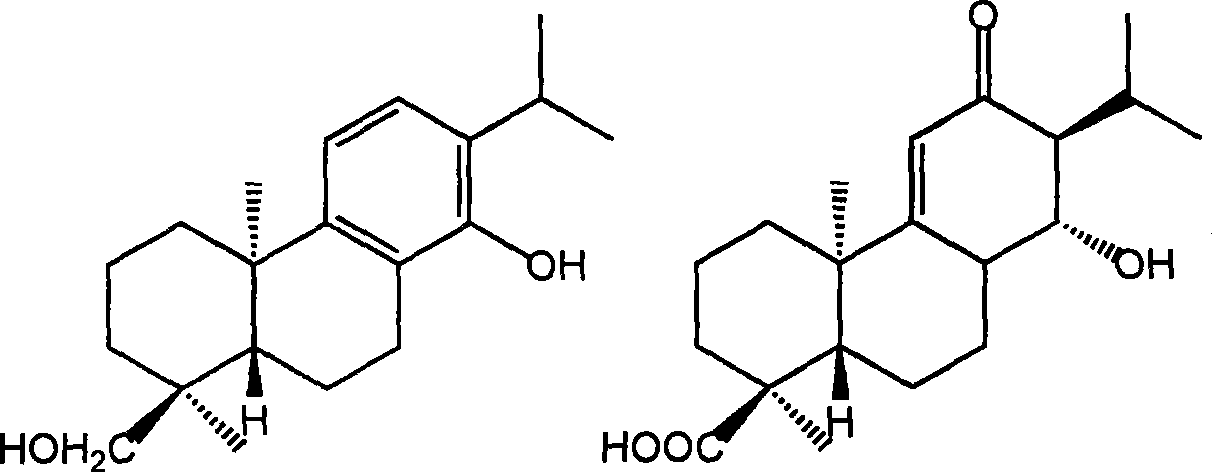
Ent-abieta diterpenoid largeleaf rabdosia leaf I and J, preparation method and use thereof
A technology of big-leaf fragrant tea and abietane, which is applied in the field of medicine to achieve obvious anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The present invention will be further described below in conjunction with the examples, but the present invention is not limited thereto.
[0024] 1 H-NMR was measured with a Varian Inova 600 instrument; MS (ESIMS and HRESIMS) was measured with a Q-TOF Micro LC-MS-MS mass spectrometer; the silica gel used was produced by Qingdao Ocean Chemical Factory; various solvents were produced by Sinopharm Produced by Group Reagent Co., Ltd., all are analytically pure.
[0025] Unless otherwise specified, the liquid / liquid ratios involved in the following examples are all volume percentages or volume ratios.
[0026] Preparation Example
[0027] Preparation of enantio-abietane-type diterpenoids and decanone
[0028] (1) Extraction: The dry weight of Dayexiangcha dish is 2kg, extract by percolating with 95% ethanol (5L) at room temperature for 3 times, the extracts are combined and then concentrated under reduced pressure, the obtained crude extract is suspended in 500ml 1N NaCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com