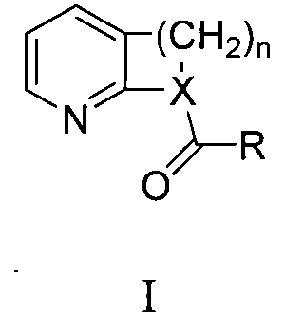
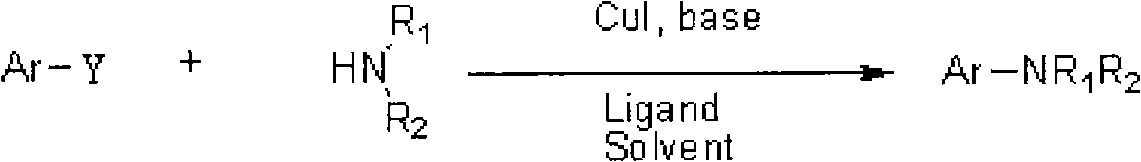Uses of 2-pyridine-beta ketone compounds
A technology of ketone compounds and pyridine, which is applied in the field of chemistry, can solve the problems of expensive palladium reagents and difficult substrate reactions, etc., and achieve the effect of novelty, cheap price and good application prospects of 2-pyridine-β-ketone compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of Ligand 2-Enteropyridine
[0034]
[0035]Under the protection of argon, add 2.96ml (32mmol) 2-picoline dropwise to 60ml THF solution dissolved with 32mmol nBuLi at 0°C, stir and react at 0°C for 45min, add 1.56ml (32mmol) acetonitrile to the reaction mixture, and stir at room temperature 3h, add 30ml*3N sulfuric acid to the reaction mixture, extract the ionic phase with diethyl ether (2*25ml), then basify with 2N sodium hydroxide solution (PH=11~12), extract with dichloromethane, and extract the organic phase with Dry over sodium sulfate, spin off the solvent under reduced pressure, and then distill the remaining oil under reduced pressure to obtain 2.2 g of the product (yield: 51%).
[0036] 1 H NMR (CDCl 3 ): δ=8.52(d, J=4.8Hz, 1H; Phenyl), 7.60(td, J=8.0, 1.6Hz, 1H; Phenyl), 7.20-7.15(m, 2H; Phenyl), 3.89(s, 2H ;CH 2 ), 2.19ppm (s, 3H; CH 3 ); 13 C NMR (CDCl 3 ): δ=205.3 (-C=O), 154.7, 149.5, 136.7, 124.1, 121.9 (Phenyl), 53.1 (CH 2 ), 29.9ppm...
Embodiment 2
[0038] Synthesis of Ligand 2-(isobutyrylmethyl)pyridine
[0039]
[0040] Synthesis method as in Example 1, yield: 50%.
[0041] 1 H NMR (CDCl 3 ): δ=8.52(d, J=4.8Hz, 1H; Phenyl), 7.60(td, J=7.6, 1.6Hz, 1H; Phenyl), 7.21-7.13(m, 2H; Phenyl), 3.96(s, 2H ;CH 2 ), 2.80-2.74 (m, 1H; CH), 1.11ppm (d, J=7.2Hz, 6H; 2CH 3 ); 13 C NMR (CDCl 3 ): δ=211.1 (-C=O), 155.1, 149.4, 136.5, 124.2, 121.9 (Phenyl), 49.9 (CH 2 ), 40.8(CH), 18.1ppm(CH 3 ); GC / MS: rt=5.91min, M / Z=163.
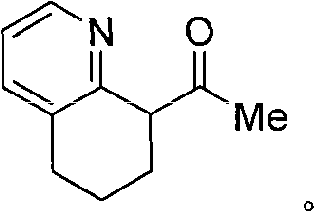
Embodiment 3
[0043] Synthesis of Ligand 8-Acetyl-5,6,7,8-Tetrahydroquinoline
[0044] Under the protection of argon, 4.26g (32mmol) 5,6,7,8-tetrahydroquinoline was added dropwise to 60ml THF solution dissolved with 32mmol nBuLi at 0°C, stirred at 0°C for 45min, and 1.56ml was added to the reaction mixture (32mmol) acetonitrile, stirred at room temperature for 3h, added 30ml*3N sulfuric acid to the reaction mixture, extracted the ionic phase with diethyl ether (2*25ml), then basified with 2N sodium hydroxide solution (PH=11~12), washed with dichloro Extract with methane, dry the organic phase with anhydrous sodium sulfate, spin off the solvent under reduced pressure, and distill the remaining oil under reduced pressure to obtain 2.3 g of the product, yield: 46%.
[0045]
[0046] 1 H NMR (CDCl 3 ): δ=8.41(s, 1H; Phenyl), 7.42(d, J=7.6Hz, 1H; Phenyl), 7.09(t, J=7.2Hz, 1H; Phenyl), 4.01(t, J=6.4Hz, 1H; CH), 2.882-2.78 (m, 2H; CH 2 ), 2.25(s, 3H; CH 3 ), 2.16-2.12 (m, 2H; CH 2 ), 1.81...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com