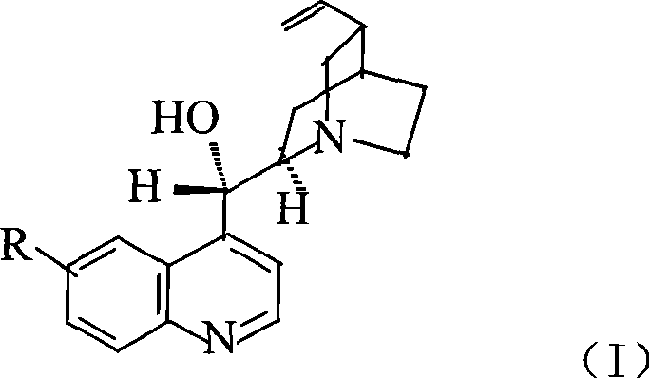
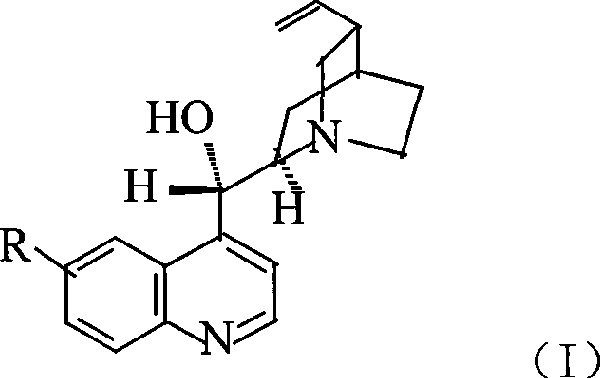
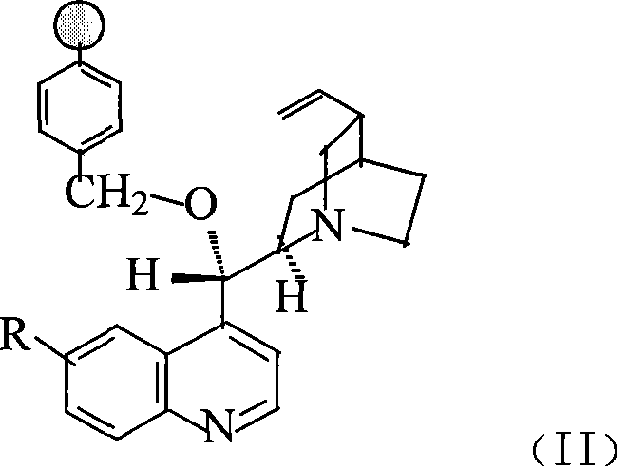
Preparation method of macromolecule loaded quinine type compound
A technology of polymer loading and cinchona alkali, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the problem of increasing the amount of solvent used, limiting, and limiting the application of catalysts Range and other issues, to achieve the effect of excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Synthesis of a macroporous cross-linked polystyrene carrier with a cross-linking degree of 10%
[0057] In a 250mL three-neck flask equipped with mechanical stirring and a condenser, add 0.3g of gelatin and 150mL of distilled water, heat and stir to dissolve the gelatin, then add 20mL of n-heptane, 18g of styrene, 2g of divinylbenzene, and 0.2g of BPO ( added to styrene). Start the mechanical stirring and control the stirring speed so that the droplet is controlled within an appropriate range. Gradually raise the temperature to 70oC, keep it warm for 2 hours, then raise the temperature to 85oC, keep it warm for 4 hours and then raise it to 95oC, keep it warm for 1 hour, filter, wash with hot water several times, extract with acetone and dry for later use. That is, a macroporous cross-linked polystyrene resin with a cross-linking degree of 10% was obtained.
Embodiment 2
[0058] Example 2 Synthesis of a macroporous cross-linked polystyrene carrier with a cross-linking degree of 6%
[0059] In a 250mL three-necked flask equipped with mechanical stirring and a condenser, add 0.3g of gelatin, 150mL of distilled water, heat and stir to dissolve the gelatin, then add 20mL of n-heptane, 18.8g of styrene, 1.2g of divinylbenzene, and 0.2 g (added to styrene). Start the mechanical stirring and control the stirring speed so that the droplet is controlled within an appropriate range. Gradually raise the temperature to 70°C, keep warm for 2 hours, then raise the temperature to 85°C, keep warm for 4 hours, then rise to 95°C, keep warm for 1 hour, filter, wash with hot water several times, extract with acetone and dry for later use. That is, a macroporous cross-linked polystyrene resin with a cross-linking degree of 6% was obtained.
Embodiment 3
[0060] Example 3 Synthesis of a macroporous cross-linked polystyrene carrier with a cross-linking degree of 50%
[0061] In a 250mL three-necked flask equipped with mechanical stirring and a condenser tube, add 0.3g of gelatin and 150mL of distilled water, heat and stir to dissolve the gelatin, then add 20mL of n-heptane, 10g of styrene, 10g of divinylbenzene, and 0.2g of BPO ( added to styrene). Start the mechanical stirring and control the stirring speed so that the droplet is controlled within an appropriate range. Gradually raise the temperature to 70°C, keep warm for 2 hours, then raise the temperature to 85°C, keep warm for 4 hours, then rise to 95°C, keep warm for 1 hour, filter, wash with hot water several times, extract with acetone and dry for later use. That is, a macroporous cross-linked polystyrene resin with a cross-linking degree of 50% was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com