Anion receptor based on nitro phenylhydrazone and phenolic hydroxyl and preparation of anion test paper and use thereof
A technology of nitrophenylhydrazone and anion, which is applied in the field of chemistry and can solve the problems of inconvenient carrying and storage of solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
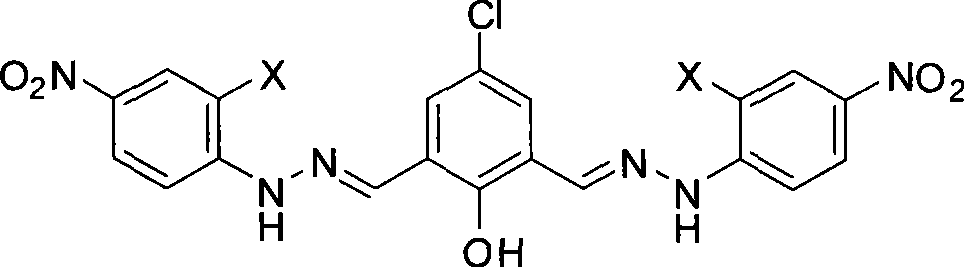
[0042] Embodiment 1, the synthesis of anion acceptor K1 (corresponding to the product of X=H)
[0043] 1. Synthesis of intermediate 2,6-dimethylol-4-chlorophenol (M1): 15 grams of p-chlorophenol, 7.8 grams of paraformaldehyde and 1.2 grams of sodium hydroxide were mixed, slowly heated to 60 ° C, 4 -Chlorophenol melts, paraformaldehyde is suspended in it, and the reactant is stirred at 60-65°C for 1 hour. Caking, the reaction is terminated. The agglomerated reaction mixture was dissolved in 100 mL of hot methanol at 70°C, and the pH was adjusted to about 2-3 with hydrochloric acid. After cooling to room temperature, the product precipitated, and was filtered to collect the product. Part of the solvent was evaporated from the filtrate and then cooled to room temperature, the product was precipitated again, and the product was combined after filtration. It was recrystallized with methanol to obtain light khaki crystals. Yield 51.0%.
[0044] 2, Synthesis of 2,6-diformyl-4-ch...
Embodiment 2、K1
[0047] The preparation of embodiment 2, K1 detection test paper
[0048] Cut the filter paper into a square of 8cm×8cm, and use 0.5mol·L -1 Soak in dilute hydrochloric acid for 1 hour. After washing with distilled water several times, filter with suction on the Buchner funnel while washing with distilled water until the filtrate is neutral. Remove the water by suction filtration, and dry the washed filter paper in a vacuum oven. Dissolve the main compound K1 in DMSO solution to prepare a concentration of 2.0×10 -2 mol L -1 DMSO solution. Put the treated filter paper flat on a 10cm plate, add the prepared DMSO solution of K1 dropwise to the center of the filter paper with a dropper, control the dropping speed, add the second drop after the first drop spreads on the filter paper, until The filter paper evenly absorbs the DMSO solution of K1. Place the test paper that has absorbed the solution in a vacuum oven to dry. After thorough drying, cut the filter paper adsorbed wi...
Embodiment 3
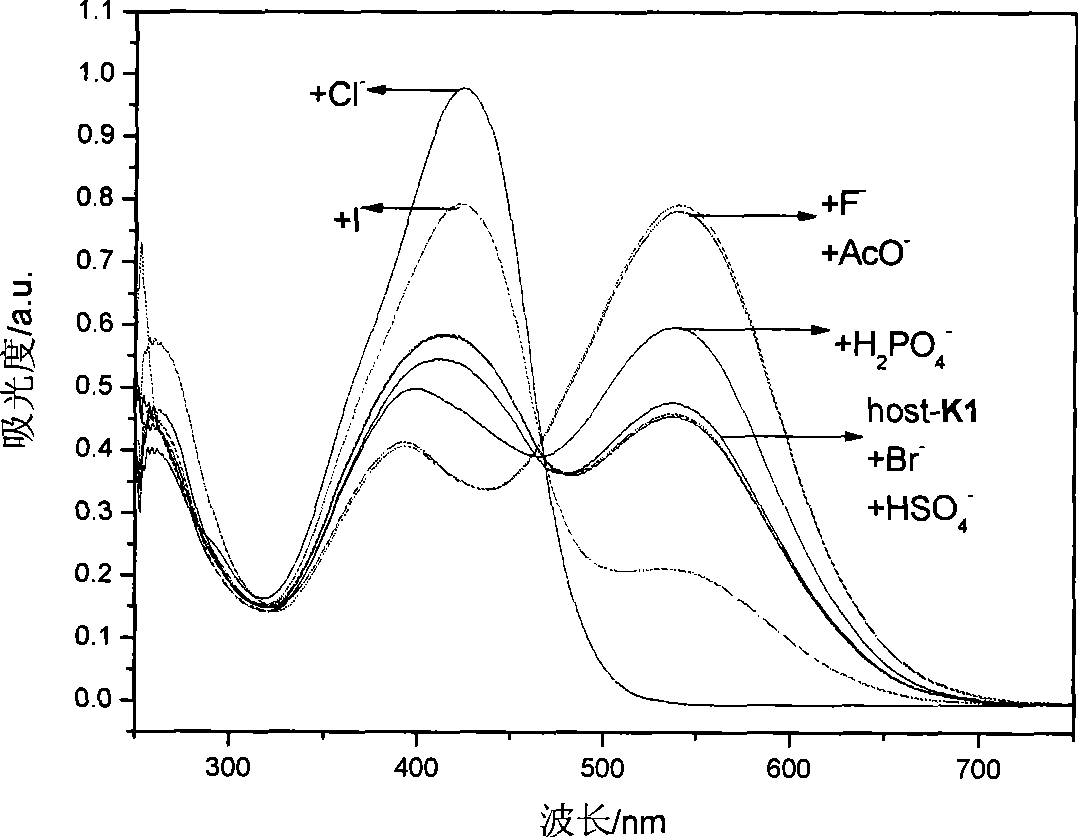
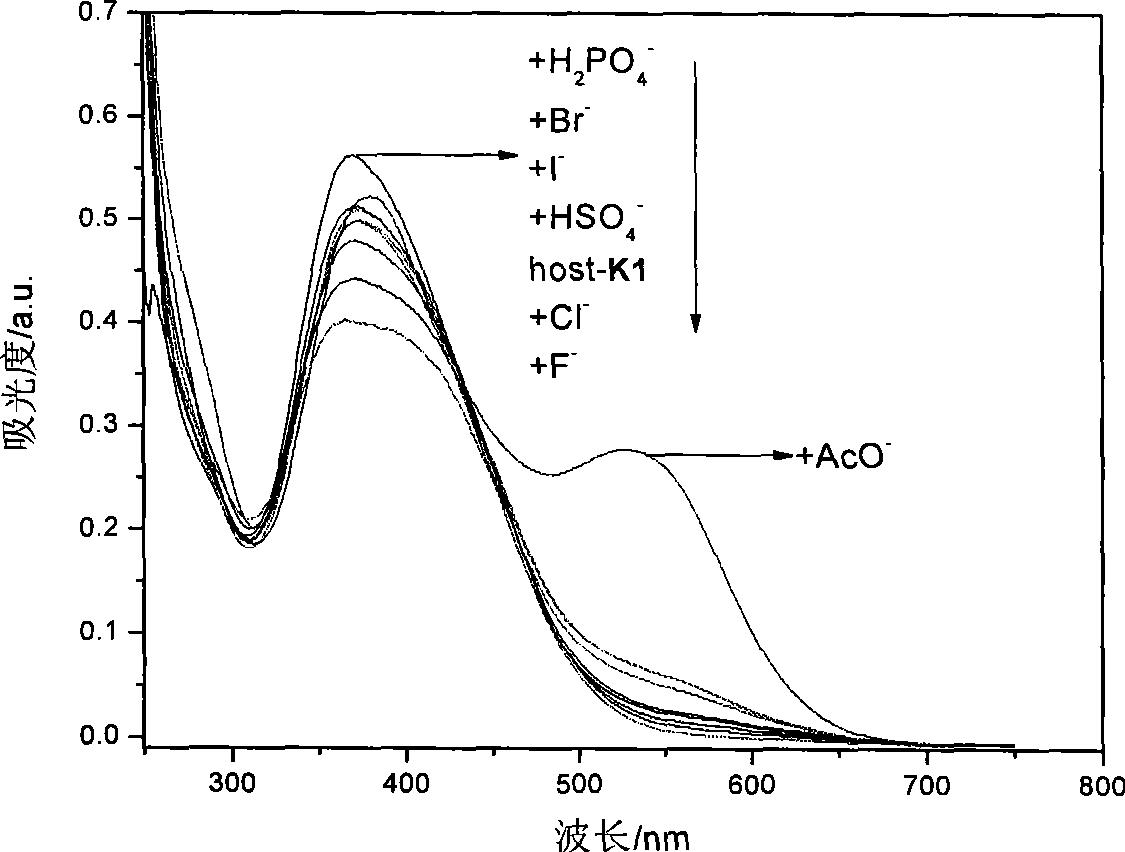
[0049] Embodiment 3, detect the F in DMSO solution with K1 detection test paper - 、AcO - and H 2 PO 4 -
[0050] will be prepared to a concentration of 1 x 10 -2 mol L -1 the F - , Cl - , Br - , I- , CH 3 COO - , HSO 4 - , H 2 PO 4 - , ClO 4 - The DMSO solution of tetrabutylammonium salt of the anion is added dropwise to the K1 detection test paper with a dropper, and the color of the test paper changes from yellow to deep red, which is F - 、AcO - or H 2 PO 4 - solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com