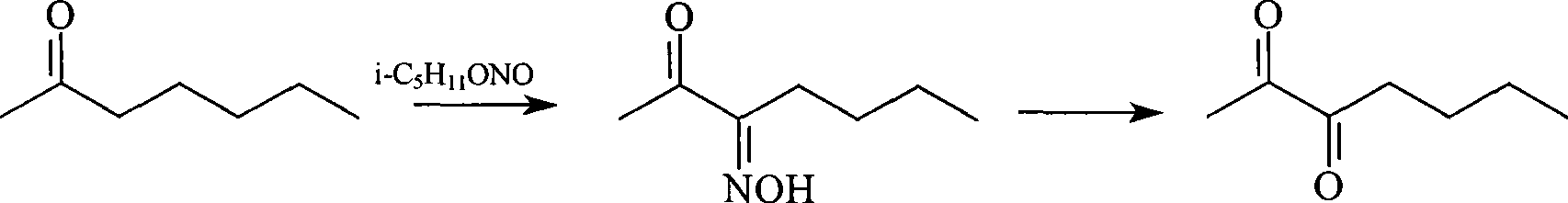
Synthetic method of 2,3-heptane dione
A technology of heptanedione and heptanedione monoxime, which is applied in the preparation of carbonyl compounds by hydrolysis and organic chemistry, can solve the problems of slow reaction speed, complicated operation and low reaction yield, and achieve fast reaction speed and high reaction yield. High, easy-to-handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0007] The following examples further illustrate the present invention.
[0008] Into a 500ml round-bottomed three-necked flask equipped with a stirrer, dropping funnel and thermometer, add 150g (1.3mol) of 2-heptanone and 12ml of dilute hydrochloric acid, and start to add 200g (1.7mol) of nitrous acid dropwise under cooling. Isoamyl ester, after dripping, continue to stir for 2 hours, the temperature is always controlled between 40-50 ℃. The reaction yield was 71.8%.
[0009] Add the 2,3-heptanedione monooxime prepared above and 2210 ml of water into a 5000 ml three-necked flask, and add a mixture of 180 g of sodium nitrite and 450 ml of water, heat and stir to 45°C, and then add 600 ml dropwise. G 30% dilute sulfuric acid solution, drip in about 1 hour. Stirring is continued for 4 hours, maintaining the reaction temperature between 50 and 60°C. Use 5% Na 2 CO 3 Solution washing, anhydrous MgSO 4 After drying, 138 grams of crude 2,3-heptanedione (content 74%) was obtained. The cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com