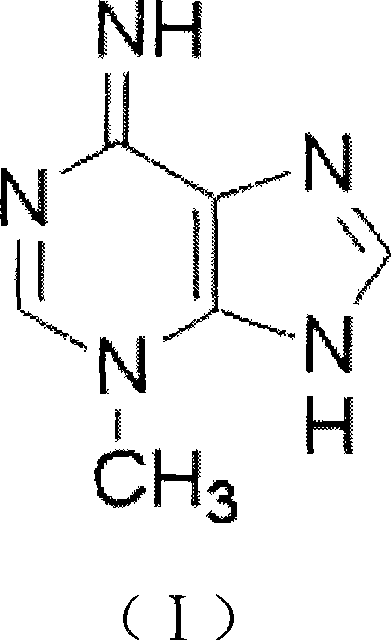
Use of 3-methyl adenine in preparing medicament for treating neurodegenerative disease
A neurodegenerative, methyladenine technology, applied in the direction of nervous system diseases, drug combinations, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
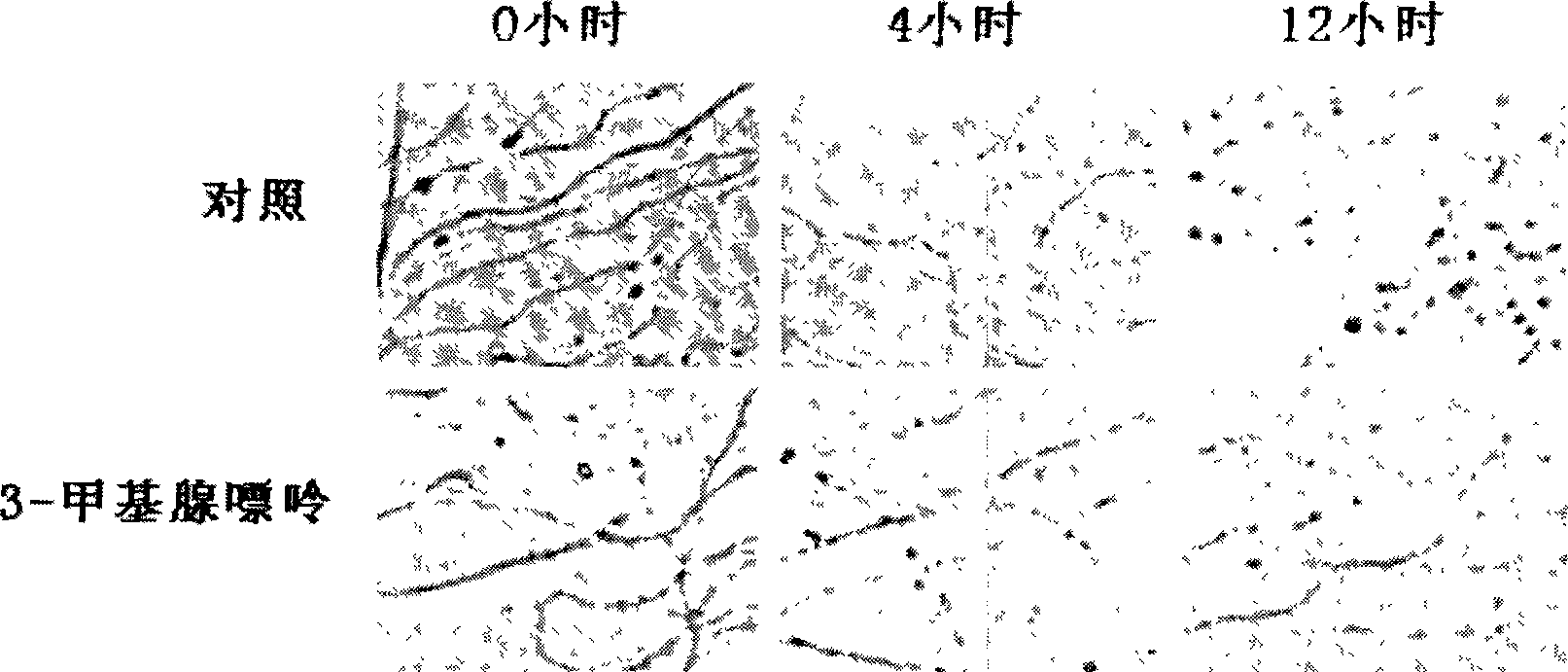
[0016] Example 1 Protection of Wallerian Axonal Degeneration Model with 3-Methyladenine
[0017] DRG ganglia isolated from E16 rat embryos were cultured in medium containing fluorodeoxyuridine and uracil to remove non-neural cells. After 5 days of in vitro culture, the axons grew longer, and the ganglion was cut along the periphery of the ganglion with a micro scalpel, and then the ganglion was removed, leaving only axons without cell bodies on the culture dish. Under normal circumstances, the axons of the neurons with the cell body removed begin to break and drop in shape after 4-6 hours, and degrade and disappear in about 12 hours. The inventors added 3-methyladenine 3 hours before removing the ganglion, and checked under a microscope whether the compound had a protective effect on neurodegeneration 12 hours after removing the ganglion. The intensity of the protective effect on axons was judged by the following quantitative method.
[0018] The quantitative analysis of axo...
Embodiment 2
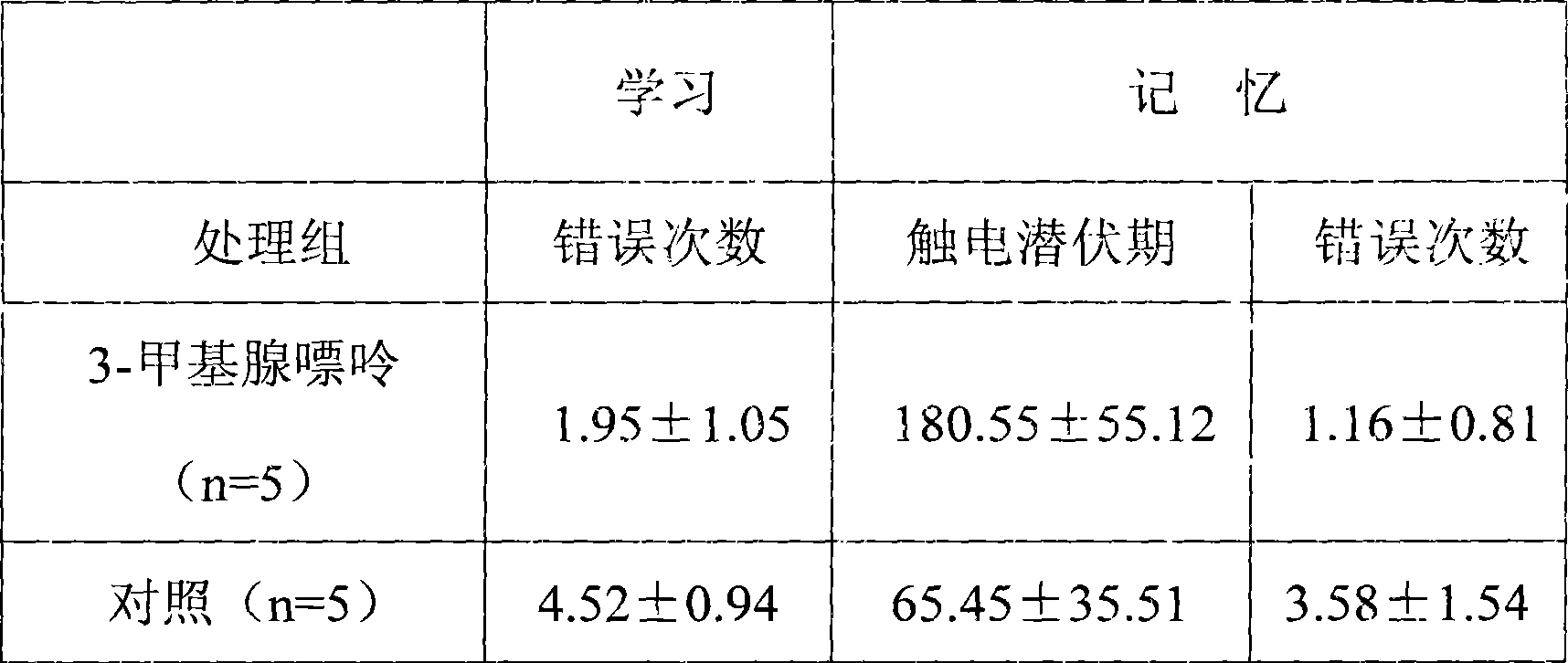
[0020] Example 2 The effect of 3-methyladenine on the behavior of model mice was detected by using a transgenic animal model of Alzheimer's disease.
[0021] Ten 8-month-old male mice of Alzheimer's disease transgenic animal model mice were randomly divided into a 3-methyladenine group (5 mice) and a control group (5 mice). The 3-methyladenine group was injected intraperitoneally with 150 mg / kg 3-methyladenine saline solution, and the control group was injected with normal saline, once every two days for 3 months. One week after the last injection, the behavioral indicators of each group of animals from the control mouse group injected with normal saline and the model group injected with 3-methyladenine were observed correspondingly, including platform jumping test and water maze test.
[0022] Platform jump test results:
[0023] Put the animal on the high platform in the experimental apparatus, electrify (AC38V) on the bottom grid, and record the number of times the mouse jum...
Embodiment 3
[0030] Example 3 Preparation of 3-methyladenine injection
[0031] Formula: 3-Methyladenine 5g
[0033] Distilled water up to 1000ml
[0034] Operation method: Dissolve the drug with physiological saline to form a solution containing 5 mg or 10 mg of 3-methyladenine per ml, and make water for injection of 5 mg or 10 mg per bottle according to the GMP requirements and the usual preparation method for water injections Injection, the finished product can be obtained after passing the quality appraisal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com