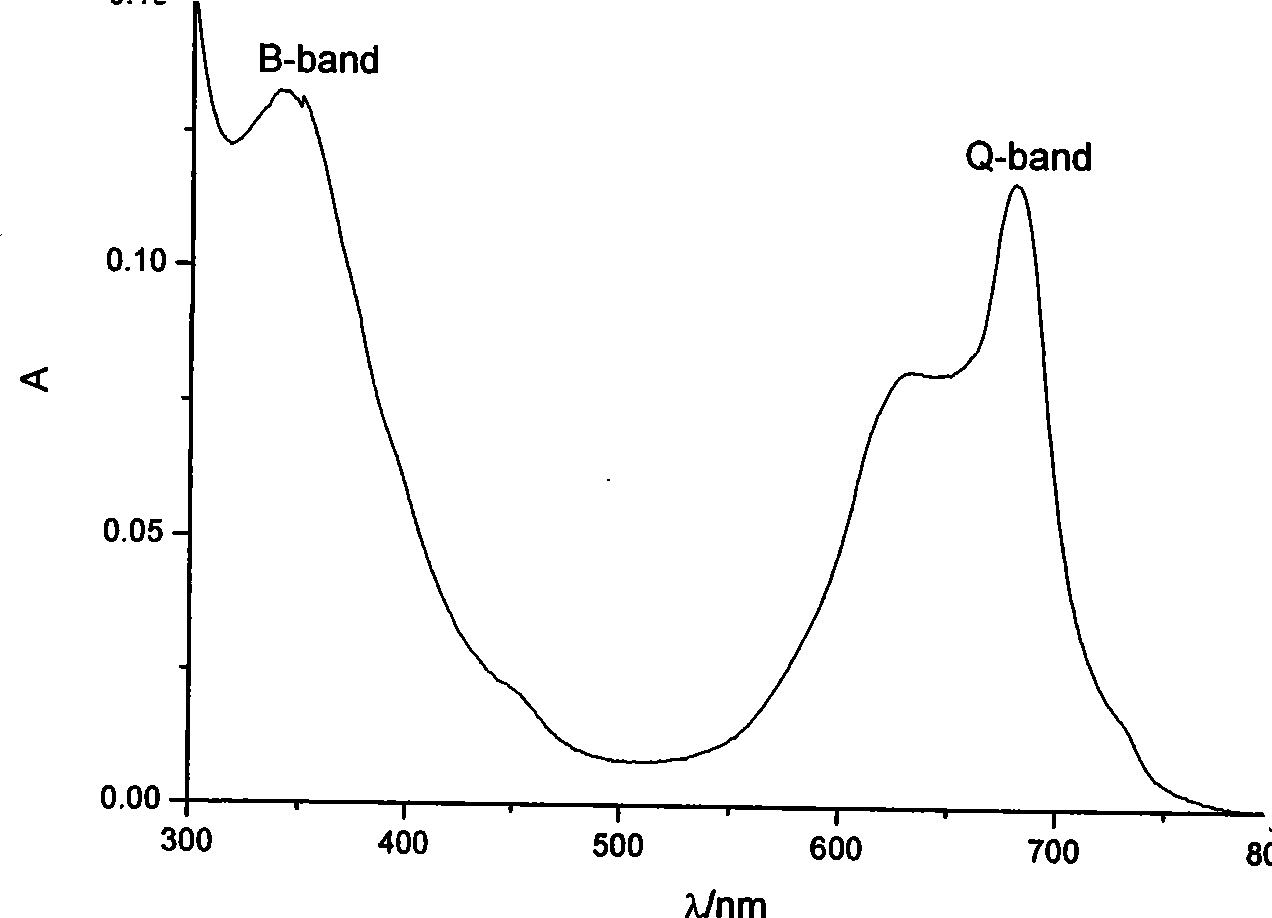
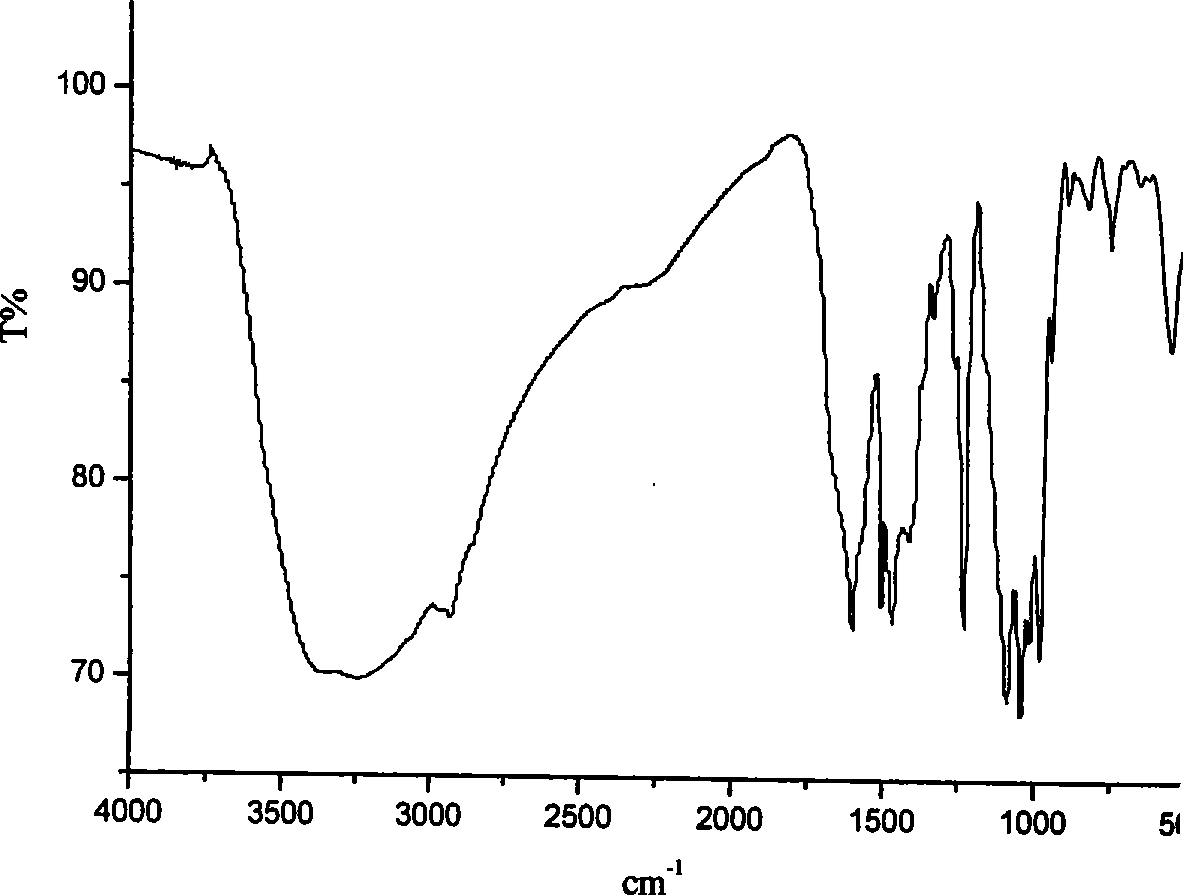
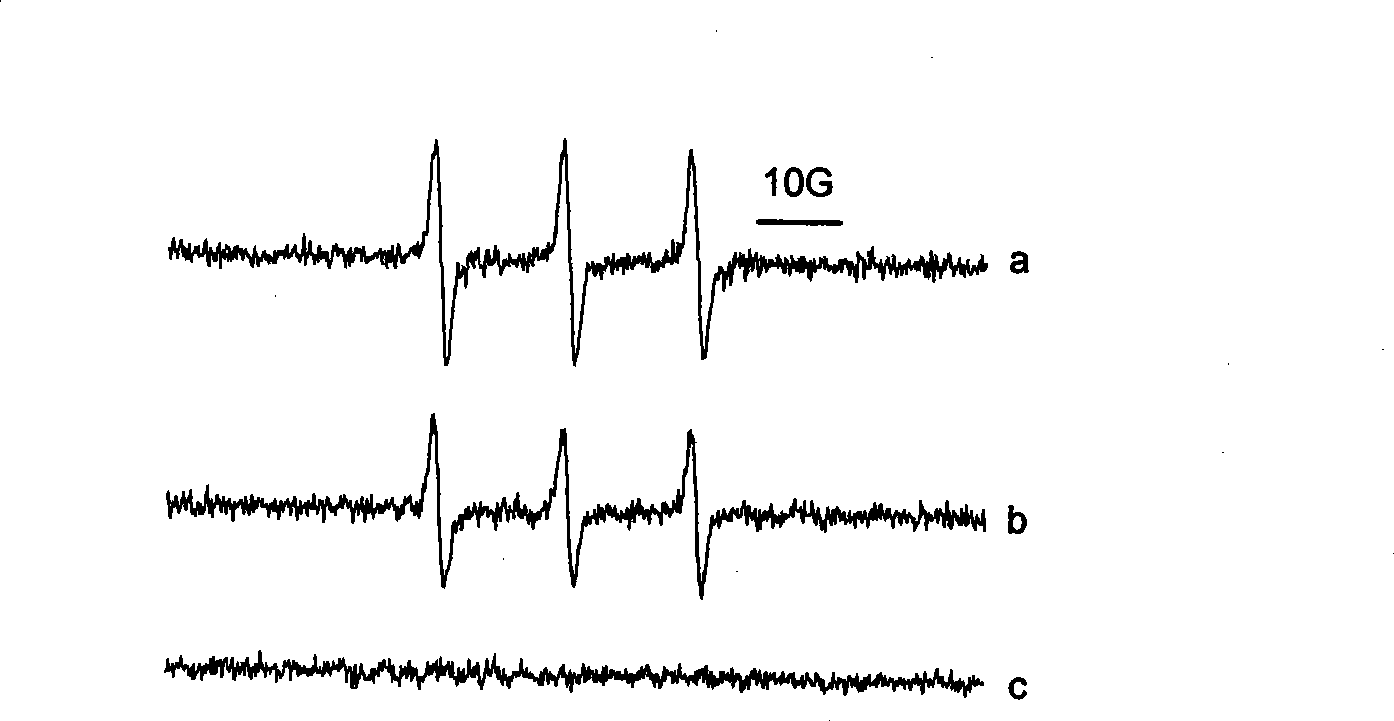
Phosphamidon amphipathic phthalocyanine derivates, preparation method and application thereof in phototherapy medicament preparation
A phthalocyanine derivative and amphiphilic technology, which is applied in the field of photosensitizers, can solve the problems of not being able to significantly reduce the dark toxicity of phthalocyanine derivatives, system stability, and limited applications, and achieve good photosensitivity, low dark toxicity, and Effects of Good Physiological Compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Phosphamide amphipathic phthalocyanine (A1) whose phosphoamido amphiphilic phthalocyanine group is connected to the phthalocyanine parent benzene ring by p-hydroxybenzaldehyde is carried out according to the following reaction pathway:
[0027]
[0028]
[0029]
[0030] Concrete synthetic steps are as follows:
[0031] (1) Add 24.2g (0.2mol) of p-hydroxybenzaldehyde, 190mL of absolute ethanol, and 12.2g (0.2mol) of ethanolamine to the reaction flask in sequence, stir at room temperature for 4-5 hours, and filter the resulting solid after the reaction, and filter cake Recrystallized from absolute ethanol to obtain a light yellow solid 2 (21.7 g) with a yield of 66.4%.
[0032] Compound Identification:
[0033] m.p.: 166-168°C. IR: (KBr, cm -1 ): 3400-2600, 3080, 1640, 1606, 1516, 1287, 1169, 1060; 1 HNMR (DMSO-d 6 ): δ 9.87(s, 1H), 8.15(s, 1H), 7.56-7.54(d, 2H, J=8.0), 6.80-6.78(d, J=8.0, 2H), 4.56(s, 1H), 3.60-3.53 (m, 4H). (2) Under an ice-water bath, ...
Embodiment 2
[0052] Example 2 is basically the same as Example 1, but the ethanolamine described in step (1) of Example 1 is replaced with propanolamine to obtain dark green solid A2.
[0053] Compound Identification:
[0054] IR: (KBr, cm -1 ): 3247, 1608, 1510, 1473, 1338, 1244, 1021, 987; 1 HNMR: (DMSO-d 6 ): δ 8.99-8.94(m, 4H), 8.57-8.50(m, 4H), 7.85-7.79(m, 4H), 7.53-7.44(m, 16H), 4.53-4.49(m, 8H), 4.32- 4.25(m, 8H), 3.59-3.50(m, 8H); 1.81-1.72(m, 8H); Anal.Calcd for C 72 h 64 N 12 Na 8 o 20 P 4 Zn: C48.30, H3.60, N9.39; Found: C47.26, H3.57, N9.36.
Embodiment 3
[0055] Example 3, the synthesis of the phosphoramido amphiphilic phthalocyanine whose phosphoamido zwitterionic group is directly connected to the phthalocyanine parent benzene ring is basically the same as Example 1, but the steps (1) and (2) of Example 1 No, directly use step 3, using ethanolamine to react with 4-nitrophthalonitrile, and the rest of the steps are the same to obtain dark green solid A3.
[0056] Compound identification: IR: (KBr, cm -1 ): 3246, 1605, 1508, 1475, 1332, 1239, 1072, 981; Anal.Calcd for C 40 h 32 N 12 Na 8 o 16 P 4 Zn: C36.68; H, 2.46; N, 12.83; Found: C36.50, H2.37, N12.67.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com