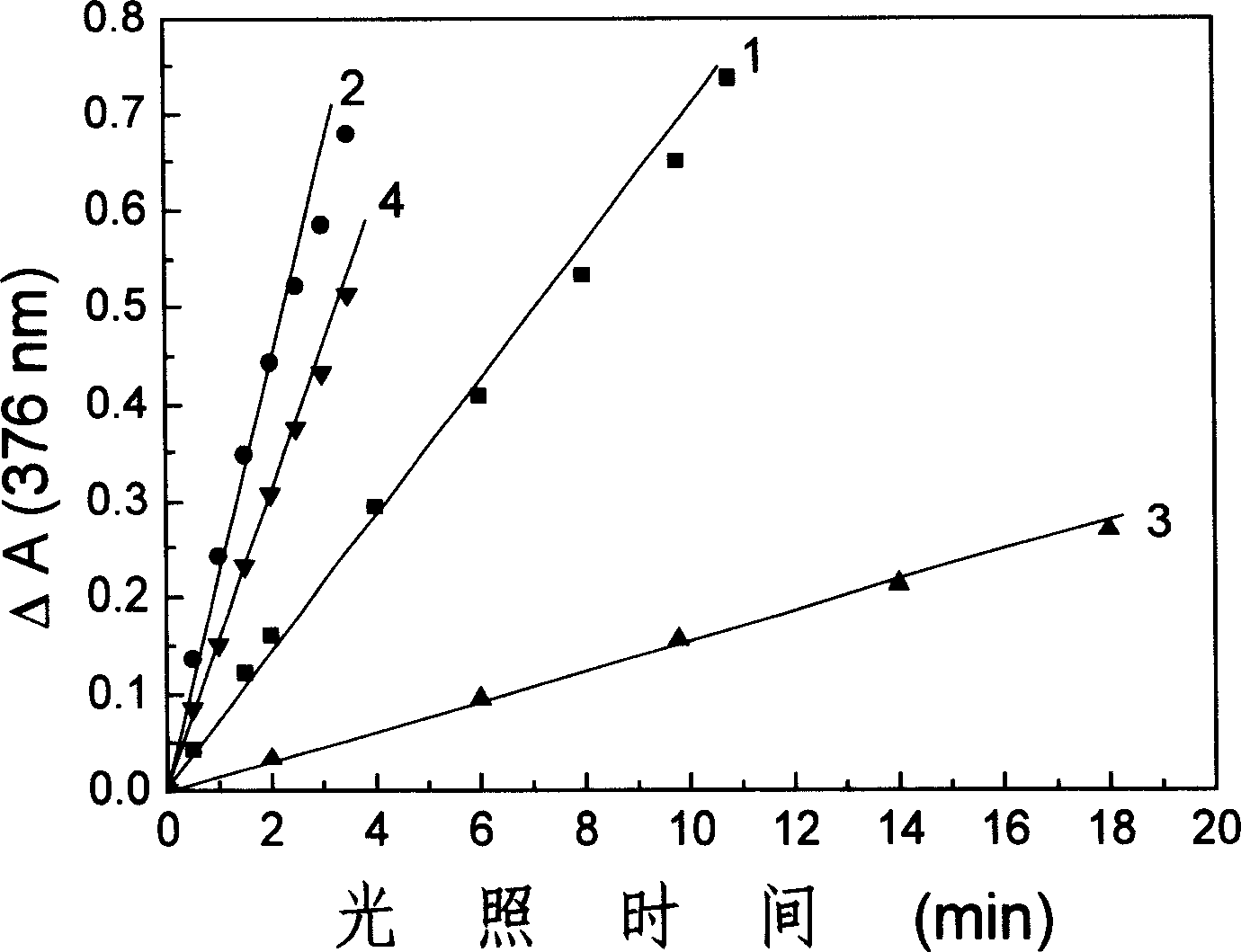
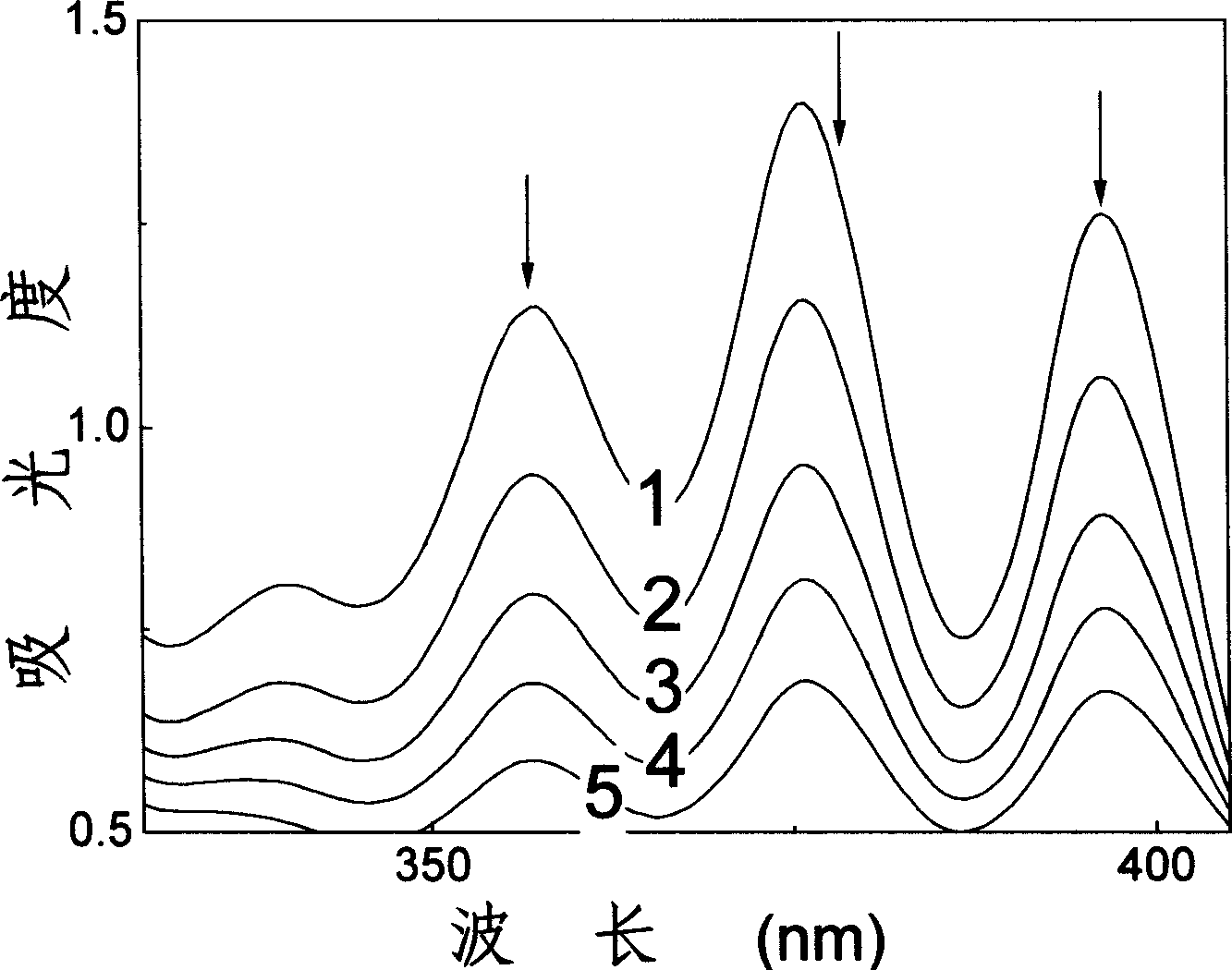
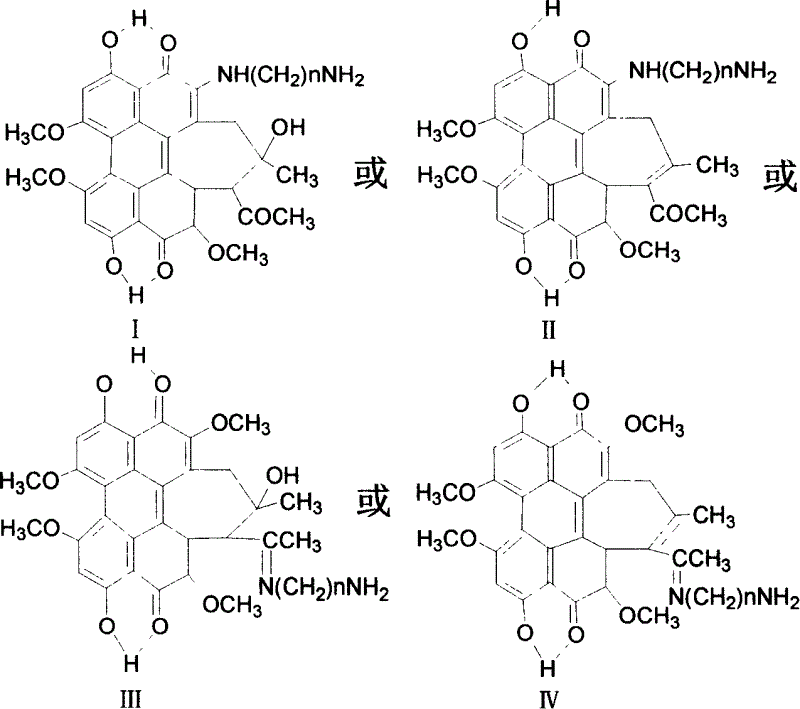
Fatty chain end group diamine substituted hypocrellin derivative and its preparing method and use
A technology of chain end-group diamine and oleocanthin, applied in the field of photosensitizers, can solve the problems of reduced singlet oxygen quantum yield, disadvantages, disadvantages, etc., and achieve the effects of low dark toxicity, good potential performance and complete structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Hypocretin B (HB) 100 mg (0.19×10 -3 mol), 50 times (molar ratio) excess 1,3-propylenediamine and 50 milliliters of tetrahydrofuran were put into a 100 milliliter three-necked round-bottomed flask, electromagnetically stirred, and reacted under argon protection for 6 hours. The solvent was evaporated under reduced pressure, the residue was extracted with chloroform for 3 to 5 times, the chloroform solution was washed with water until the water layer was colorless, the chloroform solution was concentrated under reduced pressure, separated by silica gel plate chromatography, and the developing solution was petroleum ether: ethyl acetate: ethanol , volume ratio=2:2:1. The green product components were collected and further chromatographically purified by this method to obtain the Schiff base compound of propylenediamine at position 17 and hypocrellin B.
[0027] Identification of this compound:
[0028] UV spectrum λ max : 490nm, 590nm, 635nm;
[0029] Infrared spectru...
Embodiment 2
[0036] Hypocretin B (HB) 100 mg (0.19×10 -3 mol), 50 times (molar ratio) excess 1,3-propanediamine and 100 milliliters of pyridine were put into a 250 milliliter three-necked round-bottomed flask, electromagnetically stirred, and reacted under argon protection for 13 hours. The solvent was evaporated under reduced pressure, the residue was extracted several times with chloroform, the chloroform solution was washed with water until the water layer was colorless, the chloroform solution was concentrated under reduced pressure, and separated by silica gel plate chromatography, the developing solution was petroleum ether: ethyl acetate: ethanol, volume Ratio = 2:2:1. The green product components were collected and further chromatographically purified by this method to obtain the Schiff base compound of propylenediamine at position 17 and hypocrellin B.
[0037] Identification of this compound:
[0038] UV spectrum λ max : 490nm, 590nm, 635nm;
[0039] Infrared spectrum ν max ...
Embodiment 3
[0046] Hypocretin B (HB) 100 mg (0.19×10 -3 mol), 50 times (molar ratio) excess 1,3-propylenediamine and 50 milliliters of dioxane were put into a 100 milliliter three-neck round bottom flask, electromagnetically stirred, and reacted under argon protection for 21 hours. The solvent was evaporated under reduced pressure, the residue was extracted several times with chloroform, the chloroform solution was washed with water until the water layer was colorless, the chloroform solution was concentrated under reduced pressure, and separated by silica gel plate chromatography, the developing solution was petroleum ether: ethyl acetate: ethanol, volume Ratio = 2:2:1. The green product components were collected and further chromatographically purified by this method to obtain the Schiff base compound of propylenediamine at position 17 and hypocrellin B.
[0047] Identification of this compound:
[0048] UV spectrum λ max : 490nm, 590nm, 635nm;
[0049] Infrared spectrum ν max : 33...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nuclear magnetic resonance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com