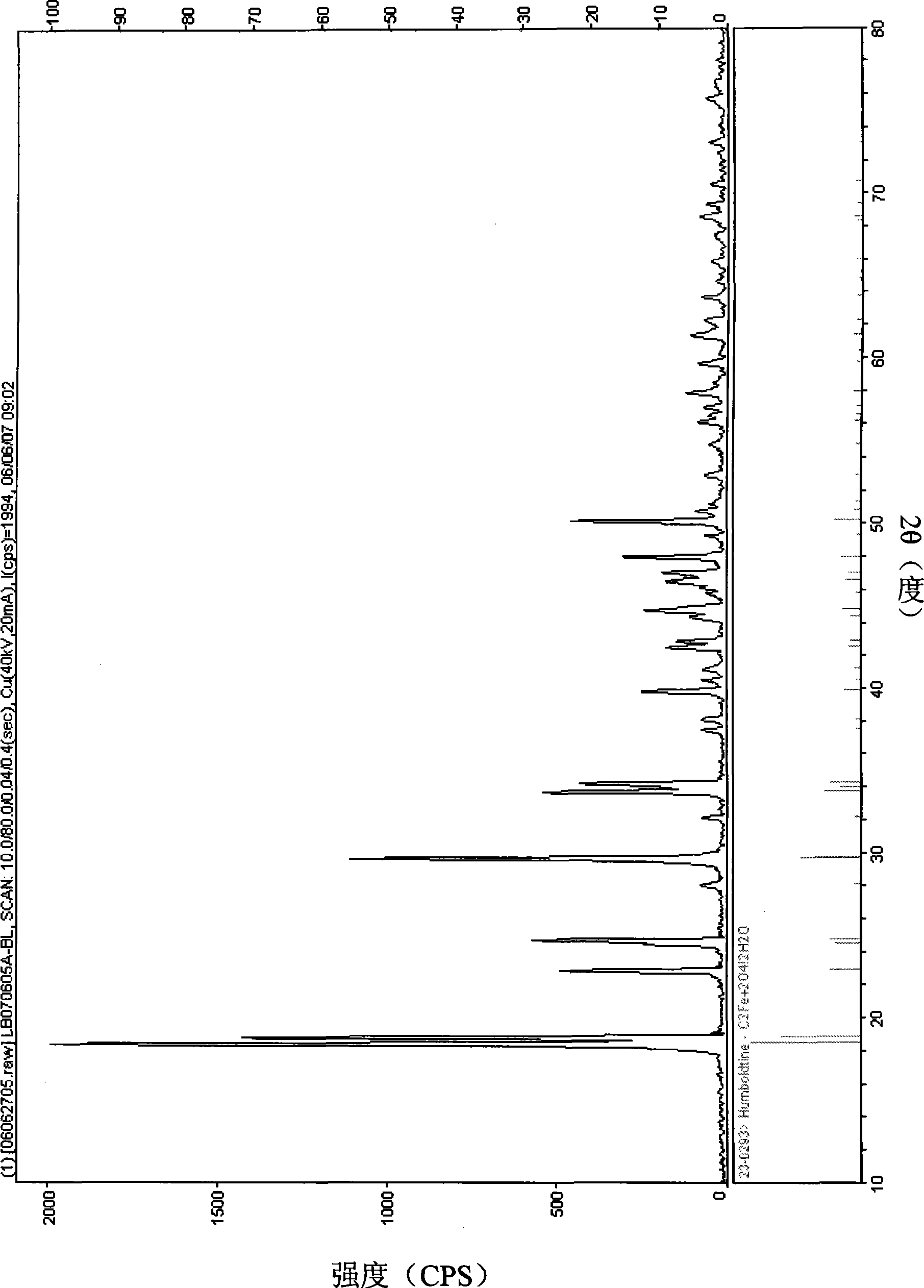
Iron oxalate crystal and preparation thereof
A ferrous oxalate and crystal technology, applied in the field of ferrous oxalate crystal and its manufacture, can solve problems such as complex process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add 4L of deionized water into a constant temperature reactor with a volume of 5L with stirring, heating and heat preservation devices, and then add 556g (2.0mol) of ferrous sulfate heptahydrate (FeSO 4 ·7H 2 O) (analytical pure), open the heating device of the constant temperature reactor and make the system temperature rise to 45 ℃, stir simultaneously and make ferrous sulfate heptahydrate dissolve. 284g (2.0mol) ammonium oxalate solid grain {(NH 4 ) 2 C 2 o 4 ·H 2 O} (analytical pure) joins in the ferrous sulfate solution that dissolves completely with the feeding rate greater than 500g / min, simultaneously with the stirring rate mechanical stirring of 500 rev / mins 20 minutes. During this process, the temperature of the system was maintained at 45°C.
[0039] Close the heat preservation device, and let it stand for 30 minutes for aging to precipitate ferrous oxalate crystals.
[0040] The supernatant was separated, and the precipitated ferrous oxalate crystals ...
Embodiment 2
[0045] Add 4L of deionized water to a constant temperature reactor with a volume of 5L with stirring, heating and heat preservation devices, and then add 1335g (4.8mol) of ferrous sulfate heptahydrate (FeSO 4 ·7H 2 O) (analytical pure), open the heating device of the constant temperature reactor and make the system temperature rise to 55 ℃, stir simultaneously and make ferrous sulfate heptahydrate dissolve. 681g (4.8mol) of ammonium oxalate solid grain {(NH 4 ) 2 C 2 o 4 ·H 2 O} (analytical pure) joins in the ferrous sulfate solution that dissolves completely with the feed rate greater than 500g / min, simultaneously with the stirring rate mechanical stirring of 800 rev / mins 120 minutes. During this process, the temperature of the system was maintained at 55°C.
[0046] Close the heat preservation device, and let it stand for 30 minutes for aging to precipitate ferrous oxalate crystals.
[0047] The supernatant was separated, and the precipitated ferrous oxalate crystals ...
Embodiment 3
[0052] Add 4L of deionized water to a constant temperature reactor with a volume of 5L with stirring, heating and heat preservation devices, and then add 1890g (6.8mol) of ferrous sulfate heptahydrate (FeSO 4 ·7H 2 O) (analytically pure), the heating device of the constant temperature reactor is opened and the system temperature is raised to 75° C., while stirring makes ferrous sulfate heptahydrate dissolve. 965.6g (6.8mol) of ammonium oxalate solid grain {(NH 4 ) 2 C 2 o 4 ·H 2 O} (analytical pure) joins in the ferrous sulfate solution that dissolves completely with the feeding rate greater than 500g / min, simultaneously with the stirring rate mechanical stirring of 1000 rev / mins 150 minutes. During this process, the temperature of the system was maintained at 75°C.
[0053] Close the heat preservation device, and let it stand for 30 minutes for aging to precipitate ferrous oxalate crystals.
[0054] The supernatant was separated, and the precipitated ferrous oxalate cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com