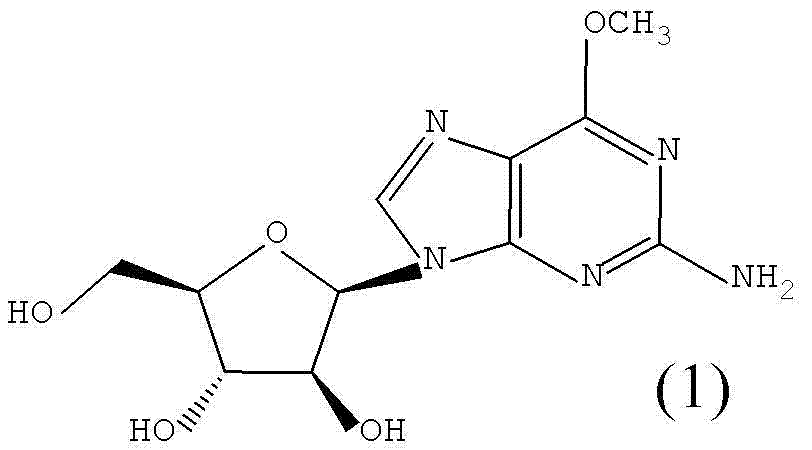
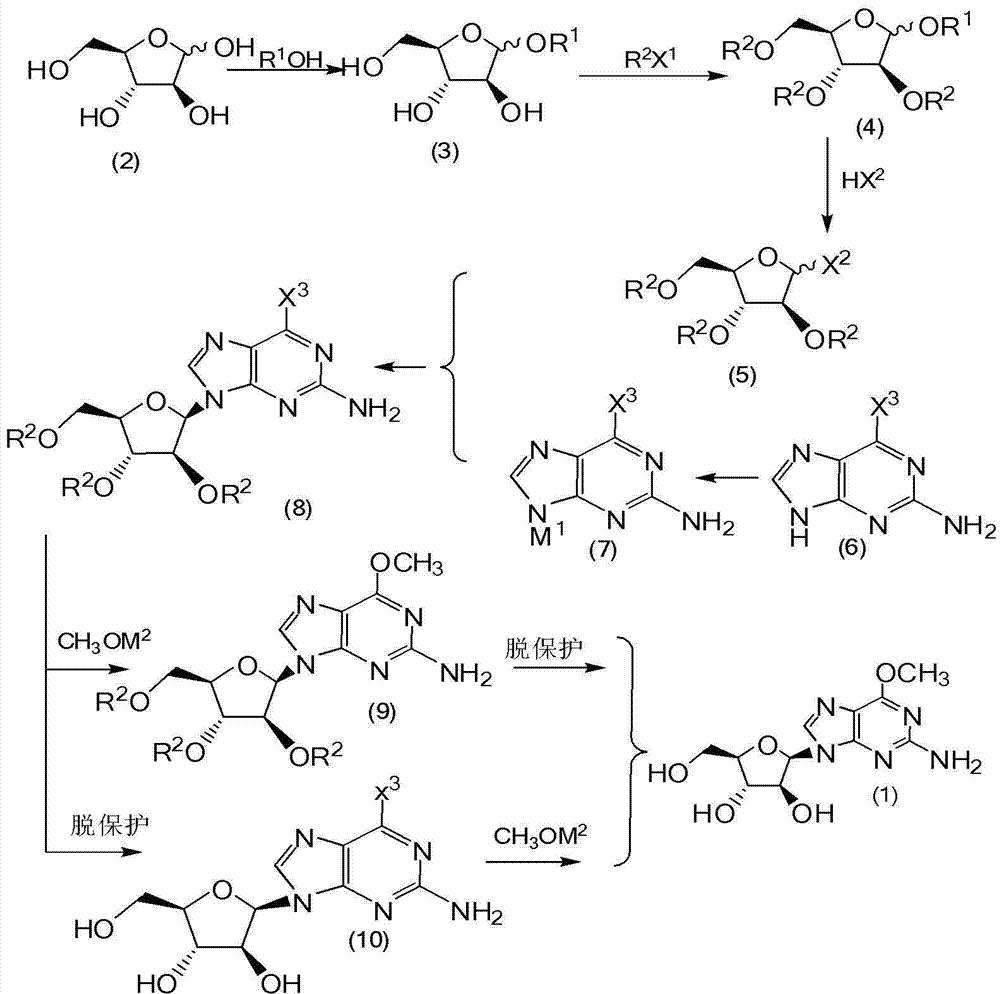
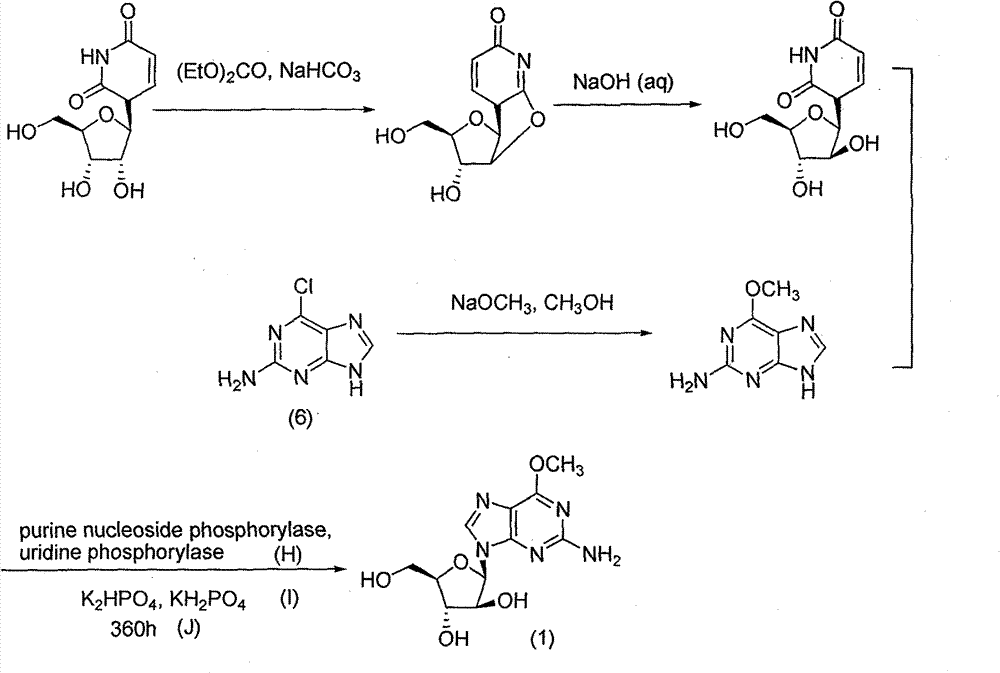
Method for synthesizing 2-amino-6-methoxy-9-(beta-D-aralino)-9H-purine
An arabinosyl and methoxyl technology, applied in the field of synthesis of 2-amino-6-methoxy-9-(β-D-arabinosyl)-9H-purine, can solve the problem of uridine phosphorylase Expensive, harsh reaction conditions, difficult to buy and other problems, to achieve the effect of simple synthesis route, mild conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of 1-oxo-methyl-D-arabinose:
[0027]
[0028] A: Add 30g (200mmol) D-arabinose to 300ml of methanol, stir to form a suspension, add dropwise 5ml of concentrated sulfuric acid, heat up to 40°C, react for 2 hours, dissolve, continue to react for 16 hours, then add 6g Potassium hydroxide precipitated a white precipitate, which was filtered by suction and concentrated to obtain 32 g of a brown-yellow sticky substance with a yield of 97%.
[0029] B:: Add 30g (200mmol) D-arabinose to 300ml of methanol, stir to form a suspension, add dropwise 5ml of concentrated sulfuric acid, react at 0°C for 48 hours, add 6g of potassium hydroxide, and precipitate a white precipitate. Suction filtration and concentration yielded 30 g of brownish-yellow sticky matter.
[0030] C:: add 30g (200mmol) D-arabinose to 300ml of methanol, stir to form a suspension, dropwise add 5ml of concentrated sulfuric acid, heat up to 68°C and reflux, after 2 hours of reaction, add 6g of potas...
Embodiment 2
[0032] Preparation of 1-oxo-(2-chloroethyl)-D-arabinose:
[0033]
[0034] Add 30g (200mmol) of D-arabinose to 200ml of 2-chloroethanol, stir to form a suspension, add 5ml of concentrated sulfuric acid dropwise, raise the temperature to 50°C, react for 2 hours, dissolve, and continue the reaction for 16 hours. 6g of potassium hydroxide was added, a white precipitate was precipitated, filtered with suction, and concentrated to obtain the product.
Embodiment 3
[0036] Preparation of 1-oxo-(2,2-dichloroethyl)-D-arabinose
[0037]
[0038]Add 30g (200mmol) of D-arabinose to 200ml of 2,2-dichloroethanol, make a suspension under stirring, add 5ml of concentrated sulfuric acid dropwise, heat up to 50°C, react for 2 hours, dissolve and continue to react for 16 After one hour, 6 g of potassium hydroxide was added to neutralize the pH to 7, and a white precipitate was precipitated, which was suction filtered and concentrated to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com