Methods and compositions for producing anti-androgenic effects
A technology of bicalutamide and composition, which can be applied in the directions of drug combination, medical preparations containing active ingredients, antitumor drugs, etc., can solve problems such as incomplete absorption, and achieve the effect of reducing the frequency of dose administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Table 3: Formulations (core tablets)
[0074] serial number
Element
quantity
quantity
(mg / tablet) 1 bicalutamide 250.00 2 lactose monohydrate 188.00 3 Sodium starch glycolate 35.00 4 polyvinylpyrrolidone 7.50 5 pure water Appropriate amount 6 Sodium starch glycolate 17.50 7 Magnesium stearate 2.00
[0075] Table 4: Formulations (Subcoat Compositions)
[0076] serial number
Element
quantity
(g / kg) 1 Hypromellose 50.00 2 Polyethylene glycol (PEG 6000) 7.50 3 Methanol 628.30 4 Dichloromethane 314.20
[0077] Table 5: Formulation (enteric coating composition)
[0078] serial number
Element
quantity
(g / kg) 1
(methacrylic acid-methyl methacrylate copolymer (1:2)) 53.85
2
(methacrylic acid-methyl methacrylate cop...
Embodiment 2
[0084] Table 6: Formulations (core tablets)
[0085] serial number
[0086] Table 7: Formulations (Subcoat Compositions)
[0087] serial number
[0088] Table 8: Formulations (Enteric Coating Compositions)
[0089] serial number
[0090] Carry out according to the same method of Example 1. Tablets corresponding to a total dose of 250 mg of bicalutamide (ie, five coated tablets) were filled into empty hard gelatin capsules.
Embodiment 3
[0092] Table 9: Formulations (core tablets)
[0093] serial number
[0094] Table 10: Formulations (Subcoat Compositions)
[0095] serial number
[0096] Table 11: Formulations (Enteric Coating Compositions)
[0097] serial number
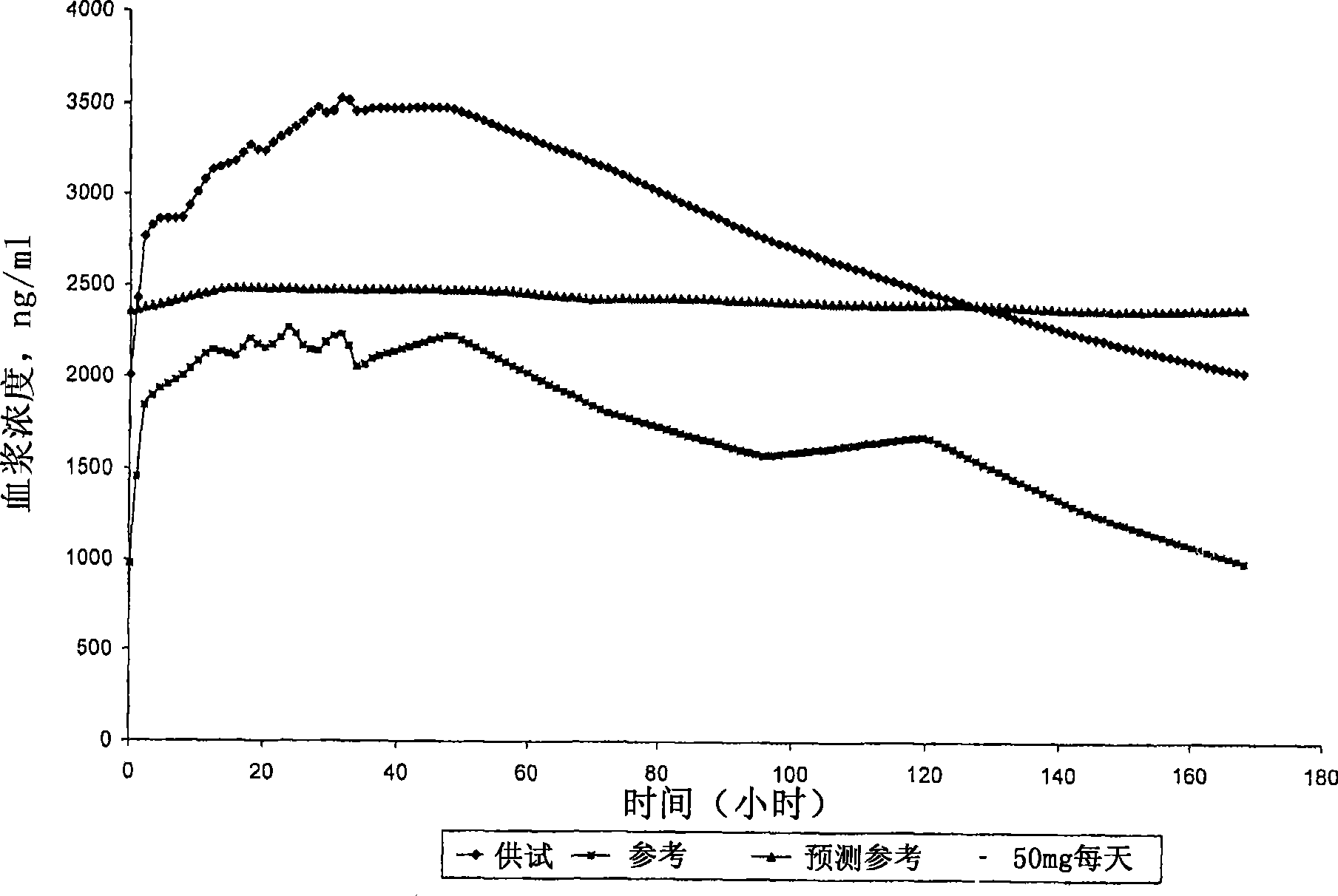
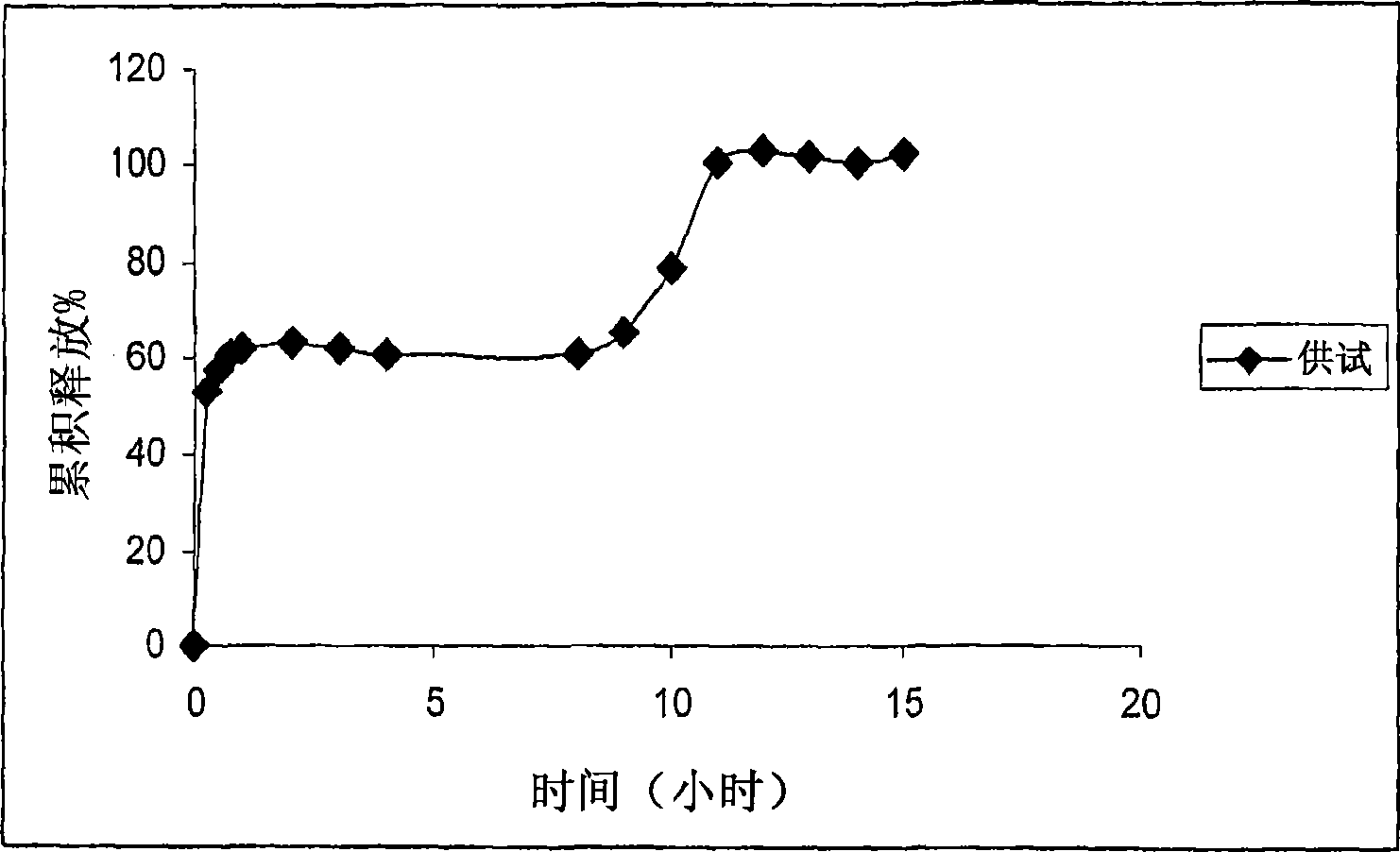
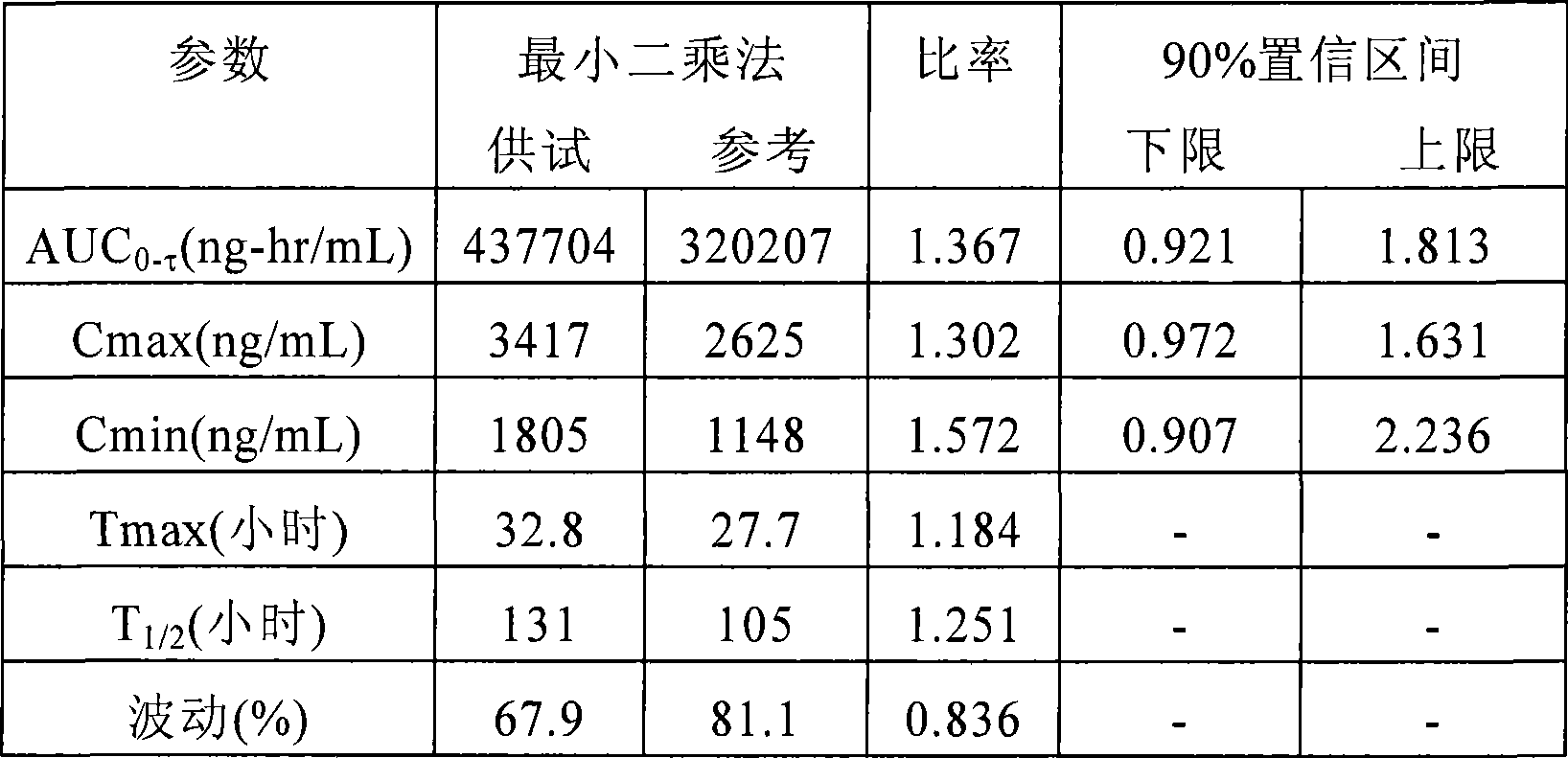
[0098] Carry out according to the same method of Example 1. Three uncoated tablets corresponding to a total dose of 150 mg and two coated tablets corresponding to a total dose of 100 mg are filled into hard gelatin capsules. Dissolution studies were performed on the compositions using the pH shift method using buffers with 1% sodium lauryl sulfate (SLS) at pH 1.2, 4.5 and 6.8. The study was performed in USPapparatus type I. The dissolution profile obtained was figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com