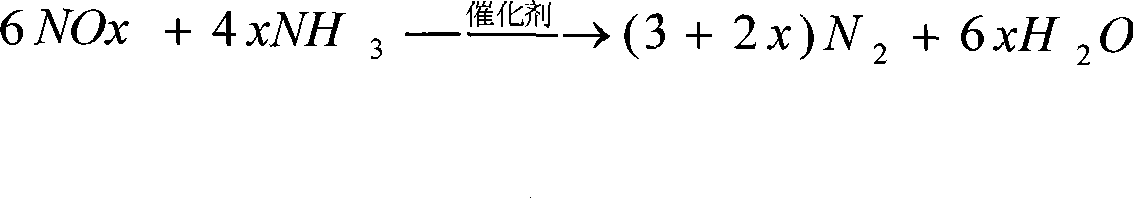
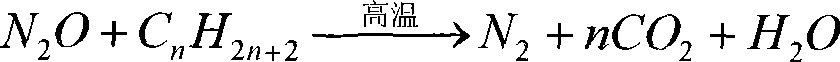Catalyst for decomposing laughing gas and preparation method thereof
A catalyst and co-catalyst technology, applied in separation methods, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of high catalyst cost, loss of activity, easy sintering, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Cordierite (400 holes / inch 2 ) after grinding and screening to obtain 40-60 mesh solid particles, after washing and pretreatment, blow-dry at 110°C for 3 hours, impregnate Al 2 o 3 The sol was used to modify the carrier, air-dried at 105° C. for 10 hours, and calcined at 650° C. for 2 hours to obtain a catalyst carrier.
[0025] Weigh cobalt nitrate [Co(NO 3 ) 2 ·6H 2 O] 9.2g, dissolved in 200mL of distilled water to obtain a cobalt nitrate solution; weigh 100g of the prepared carrier, place it in the cobalt nitrate solution and immerse it for 12.0 hours and then filter, and the filter residue was air-dried in an oven at 110°C for 3 hours to obtain a catalyst precursor I.
[0026] Weigh nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O] 21.5g was dissolved in 200mL distilled water to obtain a nickel nitrate solution; the above catalyst precursor I was placed in the nickel nitrate solution for 0.5 hours and then filtered, and the filter residue was air-dried in an oven at 110°...
Embodiment 2
[0029] Cordierite (400 holes / inch 2 ) after grinding and screening to obtain 40-60 mesh solid particles, after washing and pretreatment, blow-dry at 110°C for 3 hours, impregnate Al 2 o 3 The sol was used to modify the carrier, air-dried at 100° C. for 4.5 hours, and calcined at 600° C. for 2 hours to obtain a catalyst carrier.
[0030] Weigh cobalt nitrate [Co(NO 3 ) 2 ·6H 2 O] and [Ca(NO 3 ) 2 ·6H 2 O] 10.0g and 3.5g were dissolved in 200mL distilled water to obtain cobalt nitrate+calcium nitrate solution; 100g of the prepared carrier was weighed, placed in cobalt nitrate solution for 10 hours and then filtered, and the filter residue was placed in an oven at 95°C Blow drying for 12 hours to obtain the catalyst precursor II.
[0031] Weigh chloroplatinic acid [H 2 PtCl 6 ·6H 2 O] and nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O] 2.5g and 20.0g were dissolved in 200mL distilled water to obtain a solution containing nickel nitrate and chloroplatinic acid, and the catalyst...
Embodiment 3
[0034] Weigh cobalt nitrate [Co(NO 3 ) 2 ·6H 2 O], magnesium nitrate [Mg(NO 3 ) 2 ·6H 2 O] and zinc nitrate 8.6g, 10.4g and 6.4g, dissolve in 200mL distilled water, obtain containing cobalt nitrate and magnesium nitrate solution; Weigh the Al 2 o 3 Carrier 100g was placed in cobalt nitrate+magnesium nitrate solution, filtered after soaking for 10 hours, and the filter residue was air-dried in an oven at 95°C for 12 hours to obtain catalyst precursor III; weigh 12.8g of nickel nitrate [Ni(NO 3 ) 2 ·6H 2O], dissolved in 200mL of distilled water to obtain a nickel nitrate solution, the catalyst precursor III was placed in the nickel nitrate solution and immersed for 2.0 hours, filtered, and the filter residue was dried at 100°C for 10 hours, and roasted in a muffle furnace at 550°C for 3 hours to obtain the decomposition Laughing Gas Catalyst III.
[0035] Weigh 1g of prepared catalyst III in a fixed bed reactor, at 300°C, gas volume composition 1.35% (v)N 2 O+1.8%(v)O ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com