Use of tetrahydropyridines in preparing medicine for treating arthritis
A technology for tetrahydropyrimidine and arthritis, which is applied in the application field of tetrahydropyrimidine in the preparation of drugs for treating arthritis, and can solve the problems such as the research and report on tetrahydropyrimidine arthritis, joint degeneration, and increased infection that have not yet been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
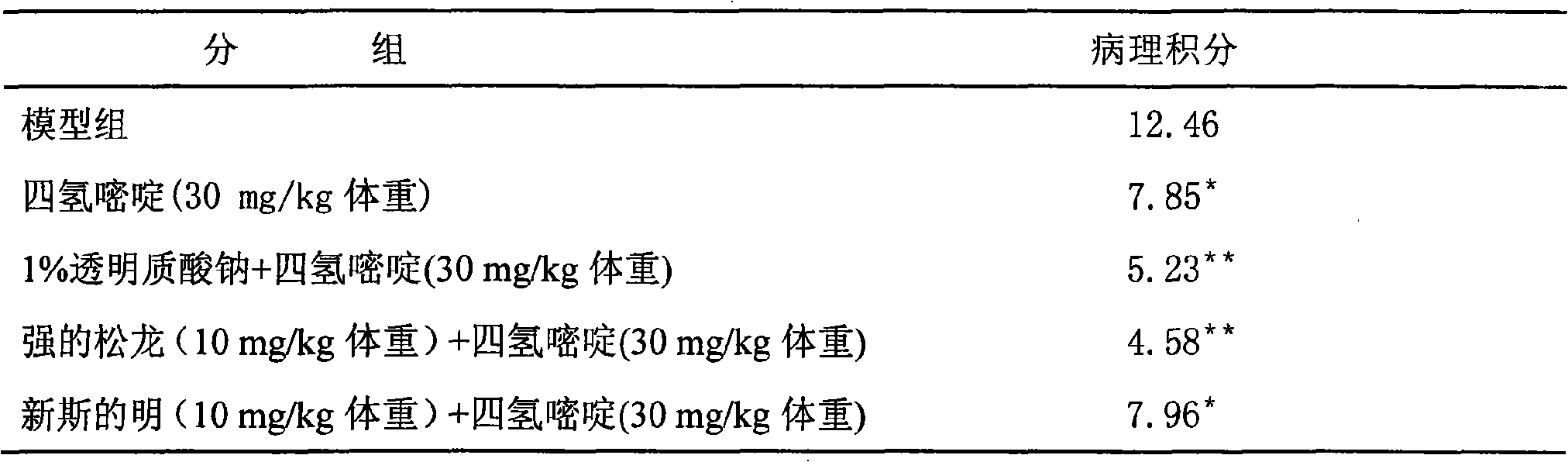
[0017] Embodiment 1: Intra-articular injection containing ectoine
[0018] Main component Ectrahydropyrimidine 2.0g
[0019] Sodium Hyaluronate 1.0g
[0020] Sulfasalazine 3.0g
[0021] Dissolve the prescribed amount of sulfasalazine in water for injection, mix and grind ectoine and sodium hyaluronate according to the prescribed amount, gradually add the above solution, adjust the pH to 6.0-8.5 with phosphate buffer, add sterile water for injection To 1000ml, adjust the osmotic pressure to 220-336 milliosm / liter. After autoclaving and subpackaging, the liquid preparation for intra-articular injection containing ectoine is obtained.
Embodiment 2
[0022] Embodiment 2: Intra-articular injection containing ectoine
[0023] Main component Ectrahydropyrimidine 3.0g
[0024] Prednisolone 1.0g
[0025] Chondroitin Sulfate 4.0g
[0026] Neostigmine 1.0g
[0027] Dissolve the prescribed amount of chondroitin sulfate in ethanol, mix and grind ectoine and prednisolone according to the prescribed amount, gradually add neostigmine and mix, dissolve with 70°C water for injection, then mix the two solutions and filter. Adjust the pH to 5.0-8.0 with phosphate buffer, add sterilized water for injection to 1000ml, and adjust the osmotic pressure to 220-336 milliosm / liter. After autoclaving and subpackaging, the liquid preparation for intra-articular injection containing ectoine is obtained.
Embodiment 3
[0028] Embodiment 3: Intra-articular injection containing ectoine
[0029] Main component Ectrahydropyrimidine 3.0g
[0030] Chitosan 2.0g
[0031] Methotrexate 2.0g
[0032] Take carbomer as the base, take 14.0g of carbomer and add water to swell, adjust the pH value to 6.0-8.0 with phosphate buffer solution, take another 2.0g of chitosan and add 80°C water for injection to swell, add the prescribed amount of tetrahydro pyrimidine, then mix the two liquids while they are hot, stir evenly, add sterilized water for injection to 1000ml, heat-press sterilize, and subpackage to obtain the intra-articular injection preparation containing ectoine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com