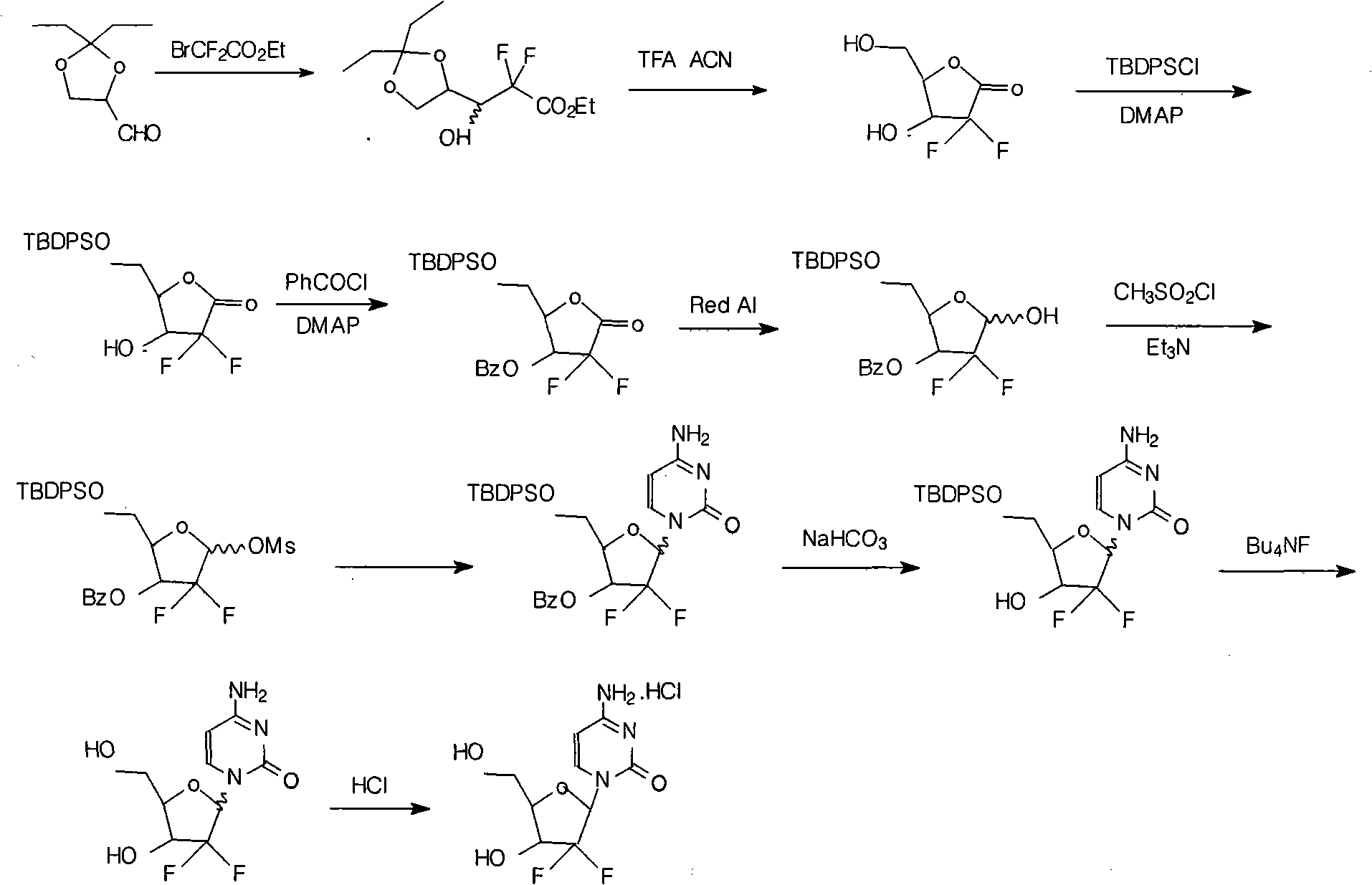
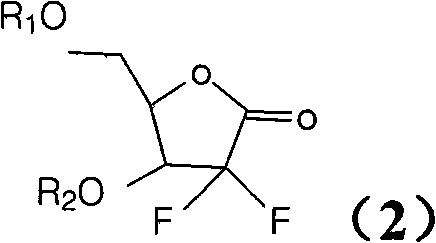
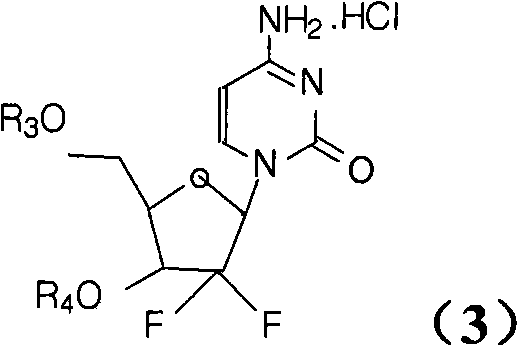
Synthesis process of the industrial production of gemcitabine hydrochloride
A technology of gemcitabine hydrochloride and synthesis process, applied in the field of synthesis technology of known compounds, can solve the problems of easy deprotection, unstable dibenzoyl protection, affecting product quality and yield, etc., and achieves improved stability and product quality. The effect of easy quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The industrial production of gemcitabine hydrochloride synthesis process is to use 2,3-oxo-isopentylidene-D-glyceraldehyde as raw material, through addition, ring opening, ring closure, and then protect the hydroxyl group with TBDPSCl and benzoyl chloride respectively. intermediate. The intermediate is reduced, mesylated, condensed with cytosine and then deprotected to form a salt to obtain gemcitabine hydrochloride.
[0022] The concrete preparation steps of its each step technique are as follows:
[0023] (1) Preparation of 3-(2,2-diethyl-1,3-dioxolyl)-2,2-difluoro-3-hydroxyl-propionic acid ethyl ester (1α / β):
[0024] Under the protection of nitrogen, put activated zinc powder (126g, 1.94mol) into the reaction bottle, THF (220ml) and stir for 10 minutes. 2,3-oxo-isopentylidene-D-glyceraldehyde (180g, 1.13mol), ethyl difluorobromoacetate (300g, 1.48mol) and THF (1000ml) were added dropwise at a temperature of 56-58°C and mixed uniformly Solution, add and keep warm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com