Manufacturing method of activated lymphocytes for immunotherapy
A technology of lymphocytes and cells, applied in the field of preparation of activated lymphocytes for immunotherapy, which can solve the problems of weak anti-cancer cell toxicity, difficulty in obtaining a large number of cells, and limitations in the clinical application of LAK cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Blood Collection and Separation of Lymphocytes
[0056] Collect 10-100ml of peripheral blood from human veins under sterile conditions. As for the blood collection container, a blood collection tube or bag containing an anticoagulant such as heparin or EDTA can be used. Then, the blood was injected into a 50ml centrifuge tube and mixed evenly with an equal amount of phosphate-buffered saline (PBS). Add Histopaque-1077 solution (Sigma) into a 50ml centrifuge tube, so that the ratio of Histopaque-1077 solution to blood diluted with PBS is 1:2-1:4. Then, slowly add blood diluted with PBS into the centrifuge tube so that the liquid level does not disperse. Then the mixture was centrifuged at room temperature at a speed of 400×g to separate the lymphocyte fraction. The section was then washed 3 times with an appropriate amount of phosphate-buffered saline. After final centrifugation and washing, the supernatant was removed, and the lymphocyte pellet was even...
Embodiment 2
[0057] Embodiment 2: the preparation of anti-CD3 antibody immobilization bottle
[0058] The antibody (Orthoclone OKT3 injection, manufactured by Ortho Biotechnology) was added to PBS to prepare a 10 ml anti-CD3 antibody solution with a concentration of 5 μg / ml, and the antibody solution was added to a culture bottle whose bottom surface area was 225 cm 2 , the solution can be evenly distributed on the bottom surface. On the next day, the antibody solution in the bottle was aspirated by a suction pump, and washed three times with PBS, thereby preparing an anti-CD3 antibody immobilized bottle.
Embodiment 3
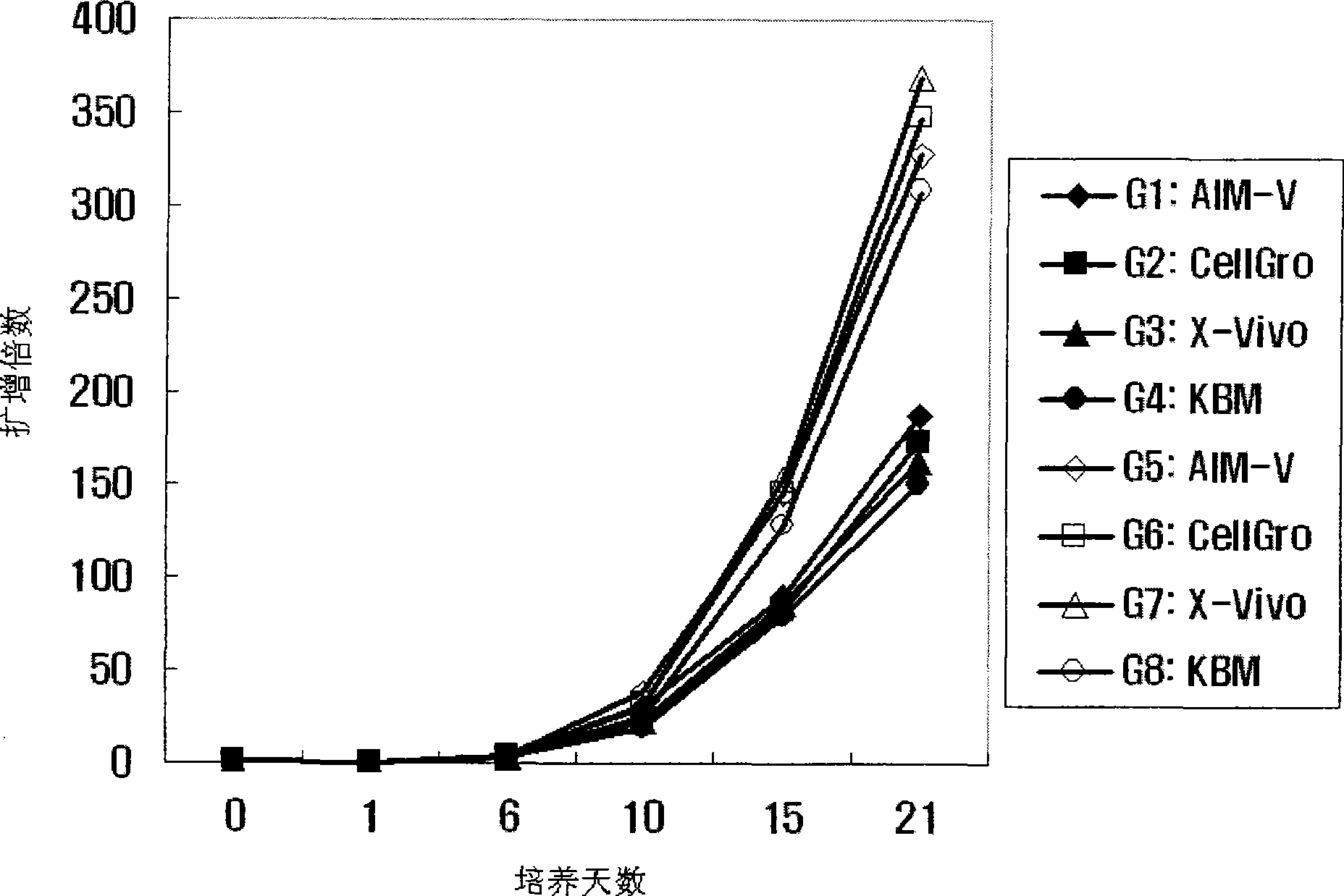
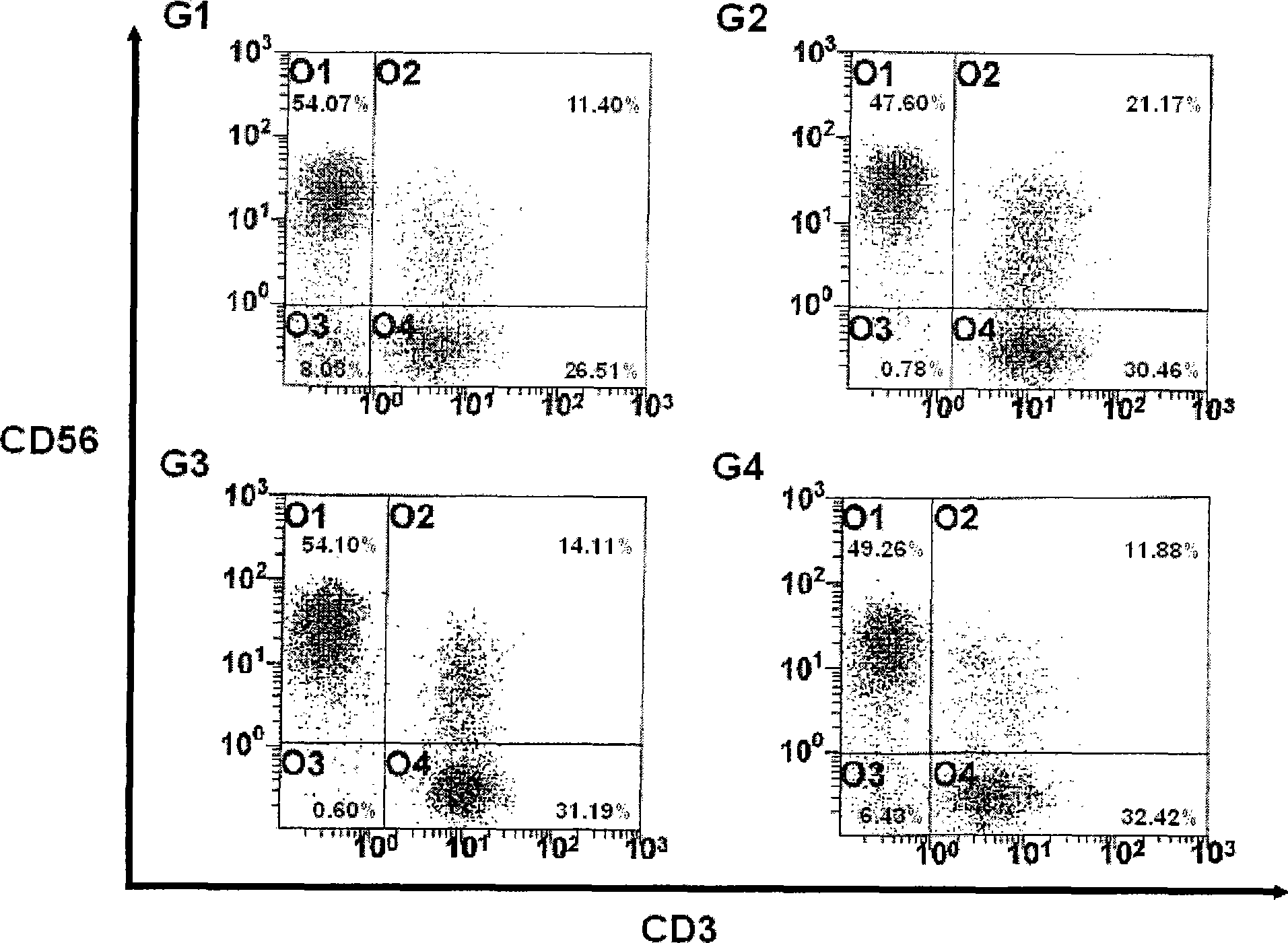
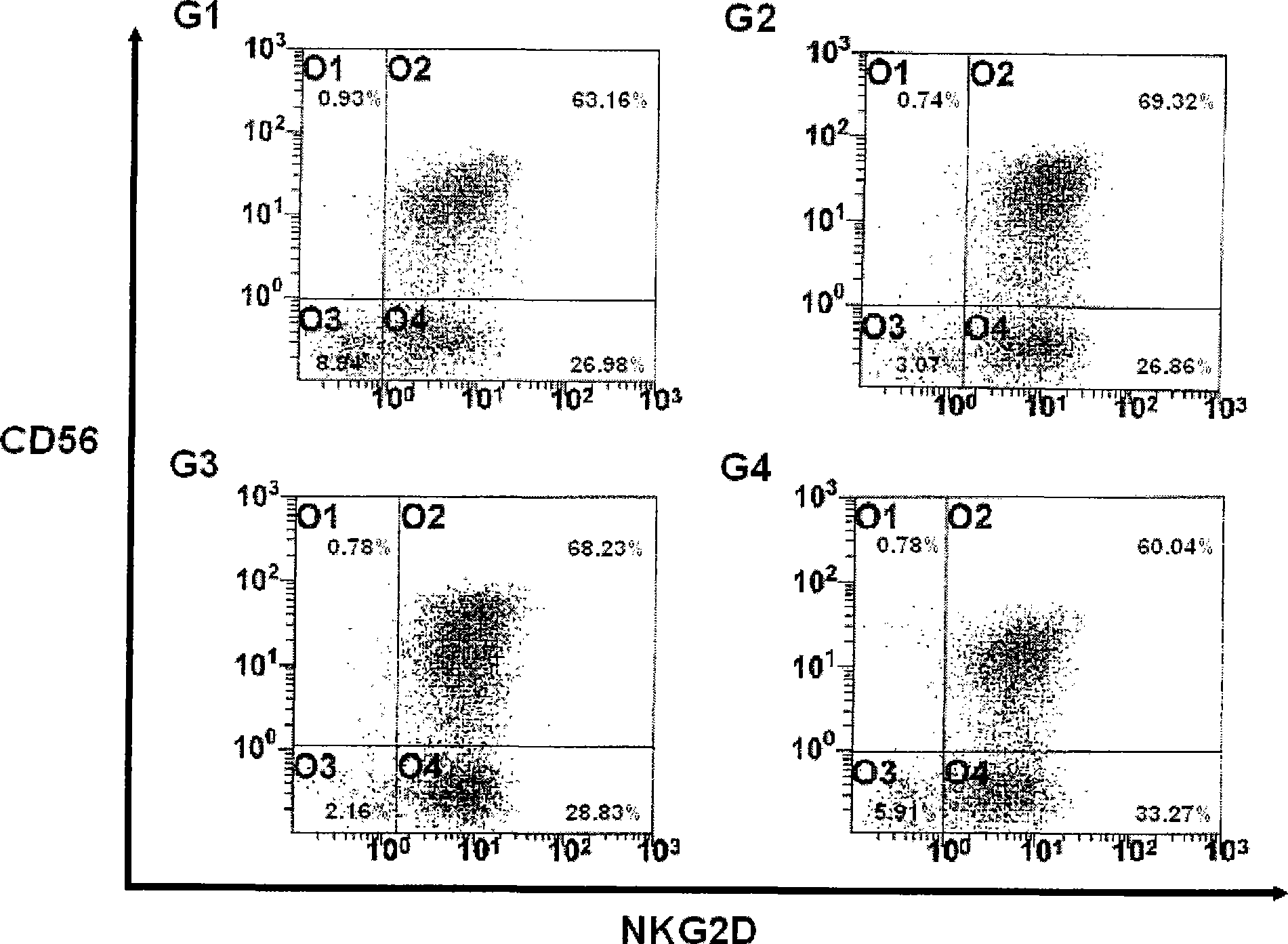
[0059] Example 3: Culture of Activated Lymphocytes
[0060] In the present invention, the proliferation rate and activation of activated lymphocytes were compared under two different culture conditions. To this end, the lymphocyte suspension was added to 50 ml of each appropriate medium (G1: AIM-V (GIBGO, USA); G2: CeIIGro (CeIIGenix); G3: KBM (Kohjin Bio); G4: X-Vivo (Cambrex ))), each medium contains 1000 U / ml IFN-γ (Leucogen, LG Life Sciences) and 1-5% human serum. Then, each medium was incubated at 37 °C and 5% CO 2 Under conditions, cultured in cell culture flasks. After 24 hours of culture, the medium in each bottle was collected and transferred to a new 225-cm 2 500 U / ml IL-2 (Proleukin, CHIRON Company) and 50 ng / ml anti-CD-30 antibody (Orthoclone, Ortho Biotechnology Company) were added to each bottle. Meanwhile, the proliferation rate and activation of cells were compared between the case of culturing with the anti-CD3 antibody-immobilized flask prepared in Exam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com