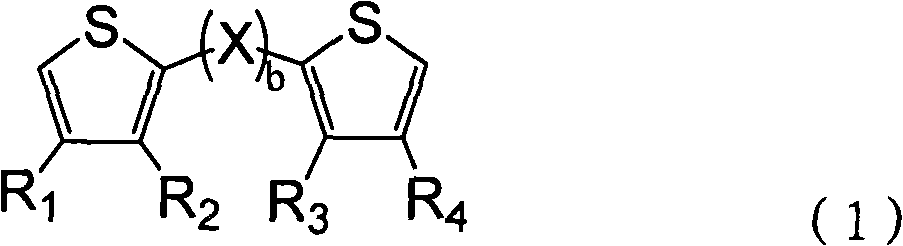
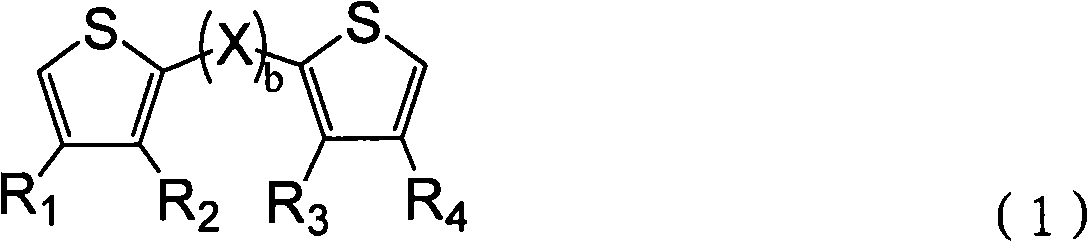Thiophene derivative and application thereof
A technology of thiophene derivatives and heterocycles, which is applied in the field of thiophene derivatives, can solve the problems of reducing the relative quantum yield and application efficiency, and achieve the effects of excellent relative quantum yield, improved solubility, and easy use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Thiophene derivatives of formula (12) were prepared in a 2 (in excess to 2.1):1 molar ratio feed according to the following procedure:
[0055]
[0056] Among them, the molecular weight of the obtained compound is 242, and its hydrogen spectrum identification position is given by 1 H NMR (solvent CDCl, 400MHz, ppm) chemical shift (δ) 7.63 (s, Ar-H 4H), 7.36-7.35 (d, Ar-H 2H), 7.31-7.30 (d, Ar-H 2H) , 7.12-7.09 (m, Ar-H 2H).
Embodiment 2
[0058] Thiophene derivatives of formula (13) were prepared in a 2 (in excess to 2.1):1 molar ratio feed according to the following procedure:
[0059]
[0060] Among them, the molecular weight of the obtained compound is 342, and its hydrogen spectrum identification position is given by 1 H NMR records (solvent CDCl 3 , 400MHz, ppm) chemical shift (δ): 7.90-7.87 (m, Ar-H 4H), 7.65-7.63 (m, Ar-H 2H), 7.43-7.41 (m, Ar-H 4H), 7.35-7.32 (m, Ar-H2H), 7.24-7.23 (m, Ar-H2H).
Embodiment 3
[0062] Thiophene derivatives of formula (14) were prepared in a 2 (in excess to 2.1):1 molar ratio feed according to the following procedure:
[0063]
[0064] Formula (14)
[0065] Among them, the molecular weight of the obtained compound is 270, and its hydrogen spectrum identification position is given by 1 H NMR records (solvent CDCl 3 , 400MHz, ppm) chemical shift (δ) 7.52 (s, Ar-H 4H), 7.24-7.23 (d, Ar-H 2H), 6.96-6.95 (d, Ar-H 2H), 2.38 (s, -CH 3 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com