Elvucitabine pharmaceutical compositions
A technology for alfcitabine and composition, which is applied in the field of alfcitabine pharmaceutical composition, can solve the problems such as the infeasibility of wet granulation method, and achieve the effect of optimizing physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] Core preparation
[0066] Eftabine-containing cores can be prepared by a variety of mixing, comminuting and preparation techniques apparent to those skilled in the art of pharmaceutical formulation. Examples of these techniques are as follows:
[0067] (1) direct tablet compression using a suitable punch and die; assemble the punch and die to a suitable rotary tablet press;
[0068] (2) injection or compression molding using a suitable mold for the compression unit; and
[0069] (3) Granulate and then compress into tablets.
[0070] When preparing the core by direct compression, the addition of a lubricant can help to facilitate powder flow when the pressure is reduced and reduce granule capping (cracking of a portion of the granule). Useful lubricants include, for example, magnesium stearate at a concentration of from about 0.05% to about 2%, preferably not more than about 1%, by weight of the powder blend. Other excipients may also be added to improve powder flow ...
Embodiment 1
[0139] Example 1. Reduction of Eftabine Particle Size
[0140] Grinding or micronization methods can be used to reduce the particle size of evetabine prior to making the tablet cores. Here, micronization refers to a method of producing smaller and more uniform particles by a micronization method other than that of producing fine particles of 5 to 10 micrometers. Micronization can be achieved by standard micronization methods for the preparation of active pharmaceutical ingredients. Micronization can be performed, for example, by a jet mill method. The parameters are as follows: injection pressure 2-8kg / cm 2 ; Micronization pressure 5-15kg / cm 2 ;Cyclone separator pressure 1-5kg / cm 2 . Particle size can be measured, for example, by laser light scattering or sieving (see US Patent No. 6,852,737, column 29, lines 31 to 45, which is hereby incorporated by reference for its teachings on micronization of active pharmaceutical ingredients).
[0141] Particle size reduction of ev...
Embodiment 2
[0145] Example 2. Optimization of Tablet Hardness
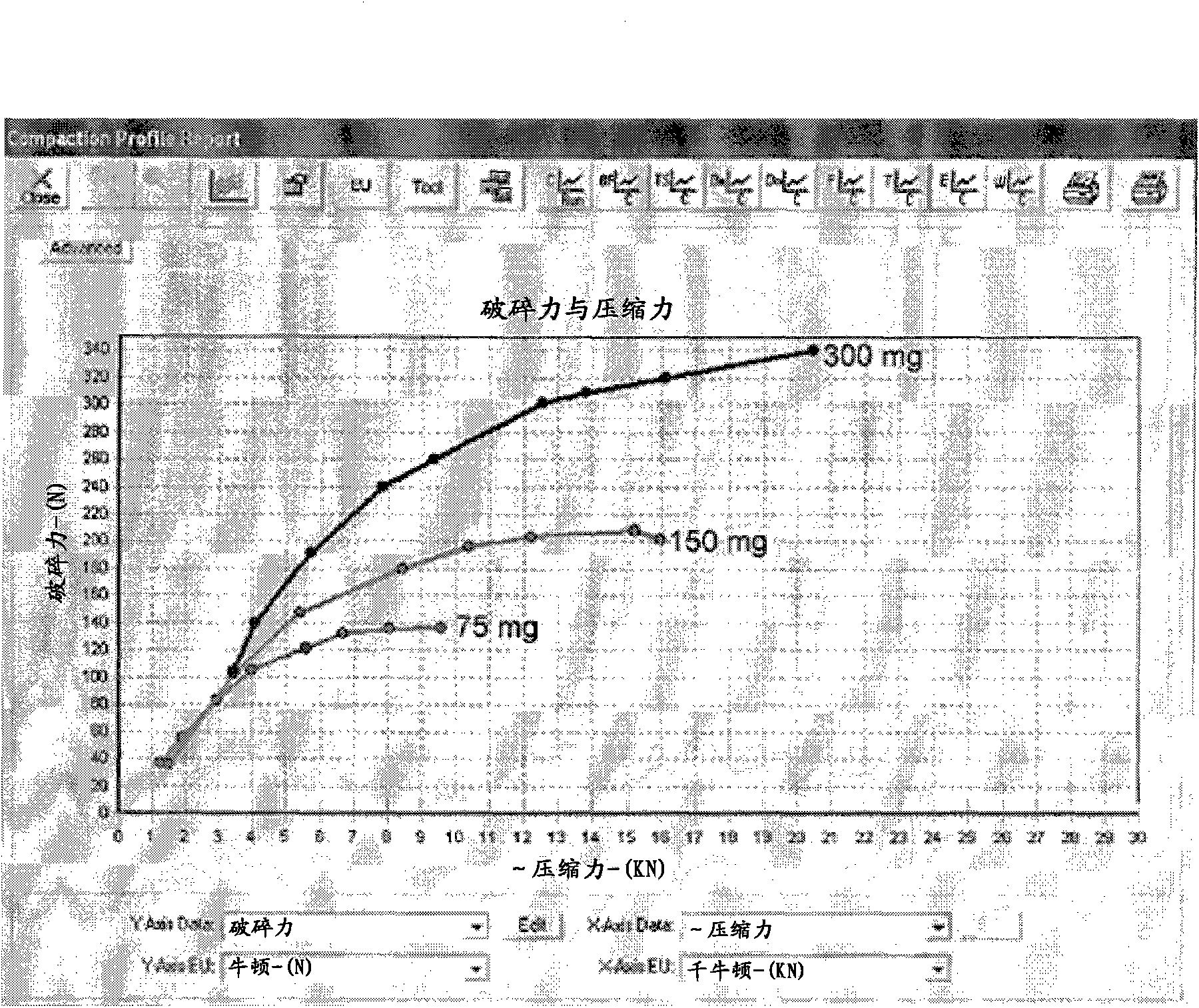
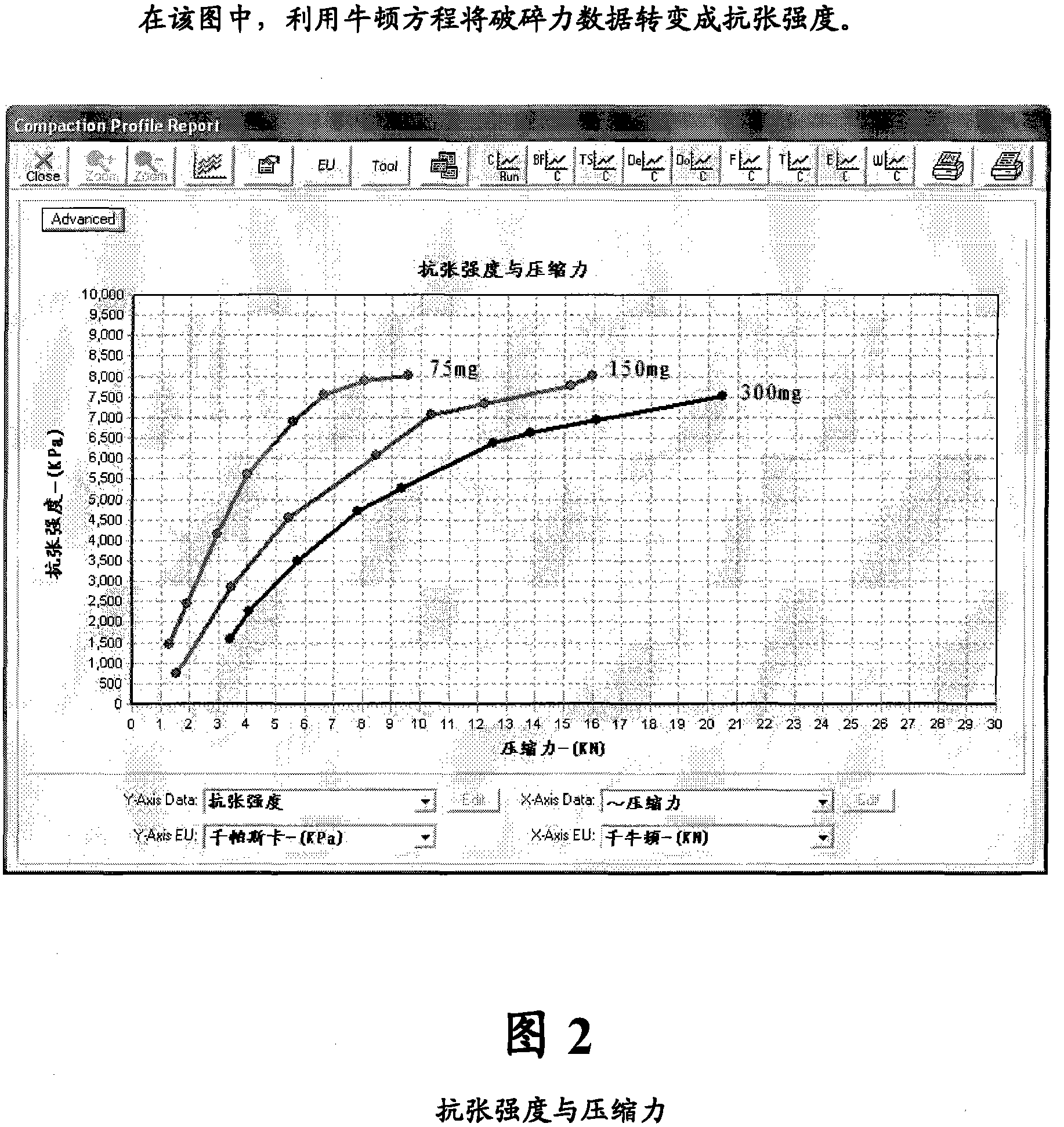
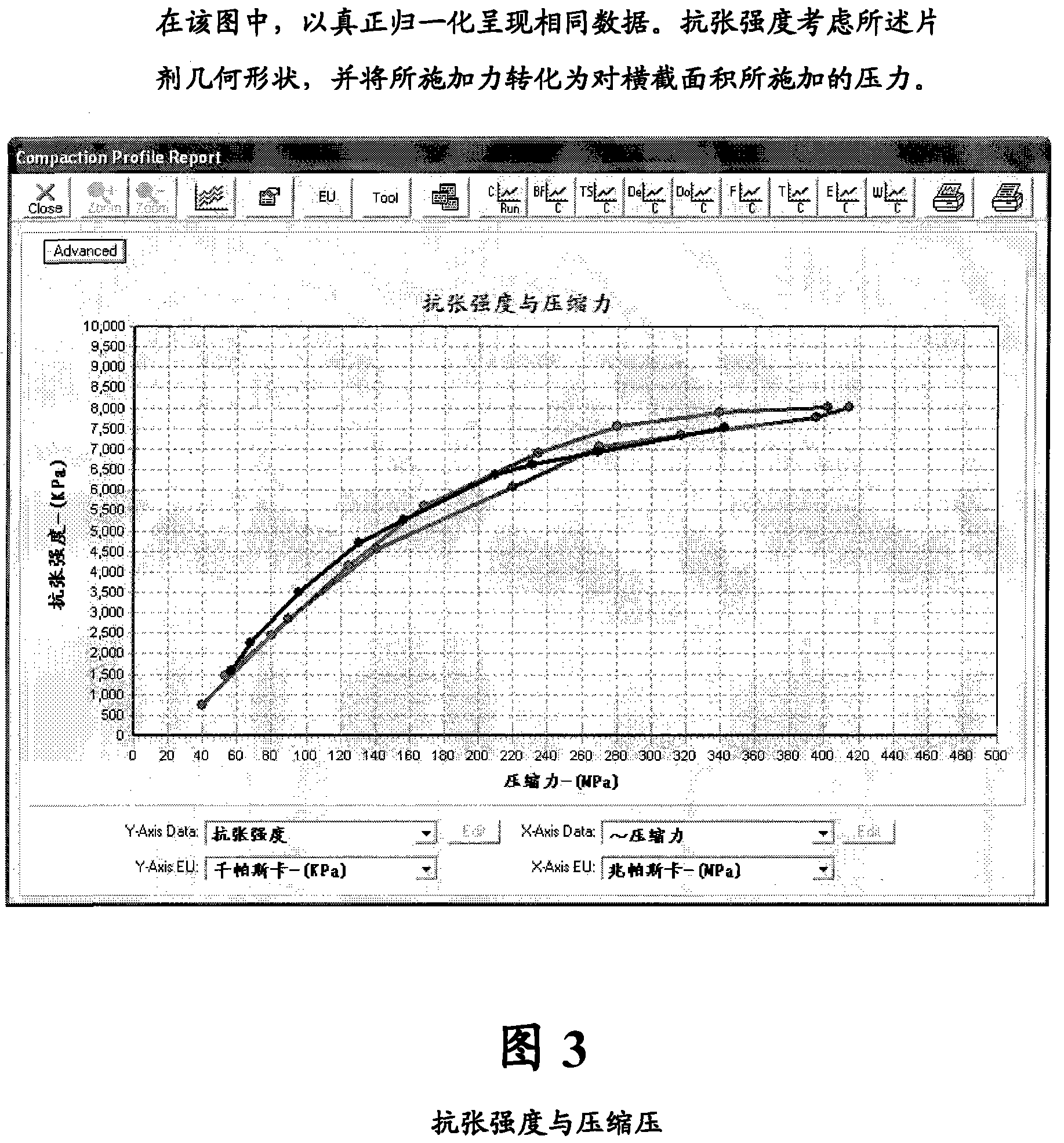
[0146] It is desired to obtain 3 different strengths of low dose tablets using a common blend. Tablets and dies were designed to give a total tablet weight representing 3 different dosage strengths of 75mg, 150mg and 300mg. A compound radius design was used on the mold as the final product will be coated. Compressive properties and strain rate studies were carried out and the results are shown graphically.
[0147] The following materials and apparatus shown in Tables 1 and 2 were used in this experiment.
[0148]
[0149]
[0150] A Riva Piccola tablet press was used in this study to determine ejection force, take-off force and compression force. The graphs and reports shown in this example were acquired and generated using the SMI Director program. The relative sizes (after coating) of the resulting tablets were obtained as part of this study.
[0151] All results are shown graphically for ease of interpretation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com