Beta lamellar blocking peptide for preventing and/or curing Alzheimer's disease
A residue and sequence technology, applied in the field of prevention and/or treatment of Alzheimer's disease, can solve the heavy burden on patients' families and society, and affect the quality of life of patients and their family members, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] Preparation of the polypeptide of the present invention
[0070] The polypeptides of the present invention can be prepared by any method known to those skilled in the art for preparing polypeptides.
[0071] Polypeptides of the invention can be synthesized using chemical synthesis. Polypeptide synthesis can be performed in solution or using solid-phase synthesis. The solid-phase synthesis method of polypeptide includes Fmoc solid-phase synthesis method and tBoc solid-phase synthesis method. The method of artificially synthesizing polypeptides is generally synthesized from the C-terminus (carboxyl-terminus) to the N-terminus (amino-terminus).
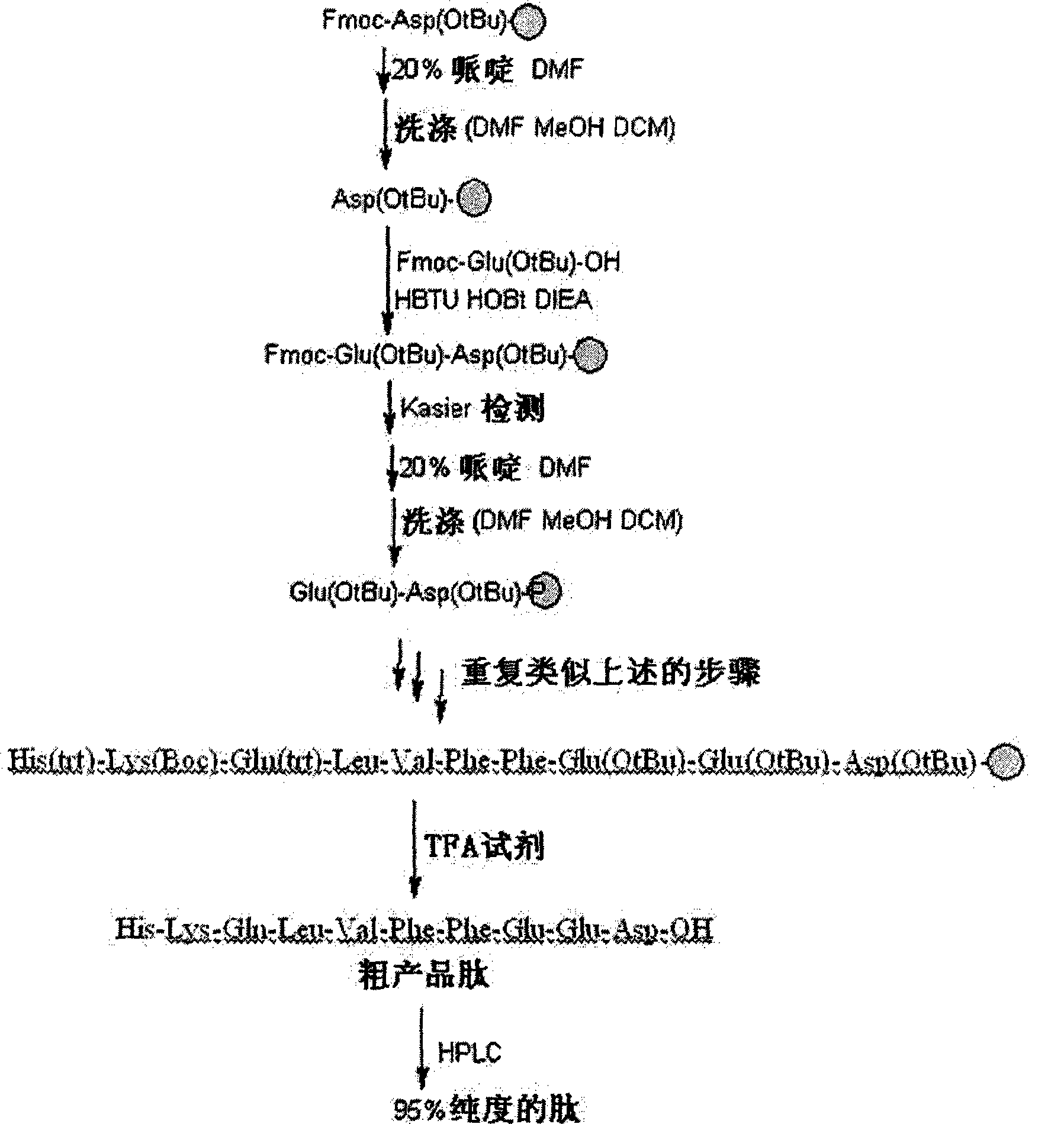
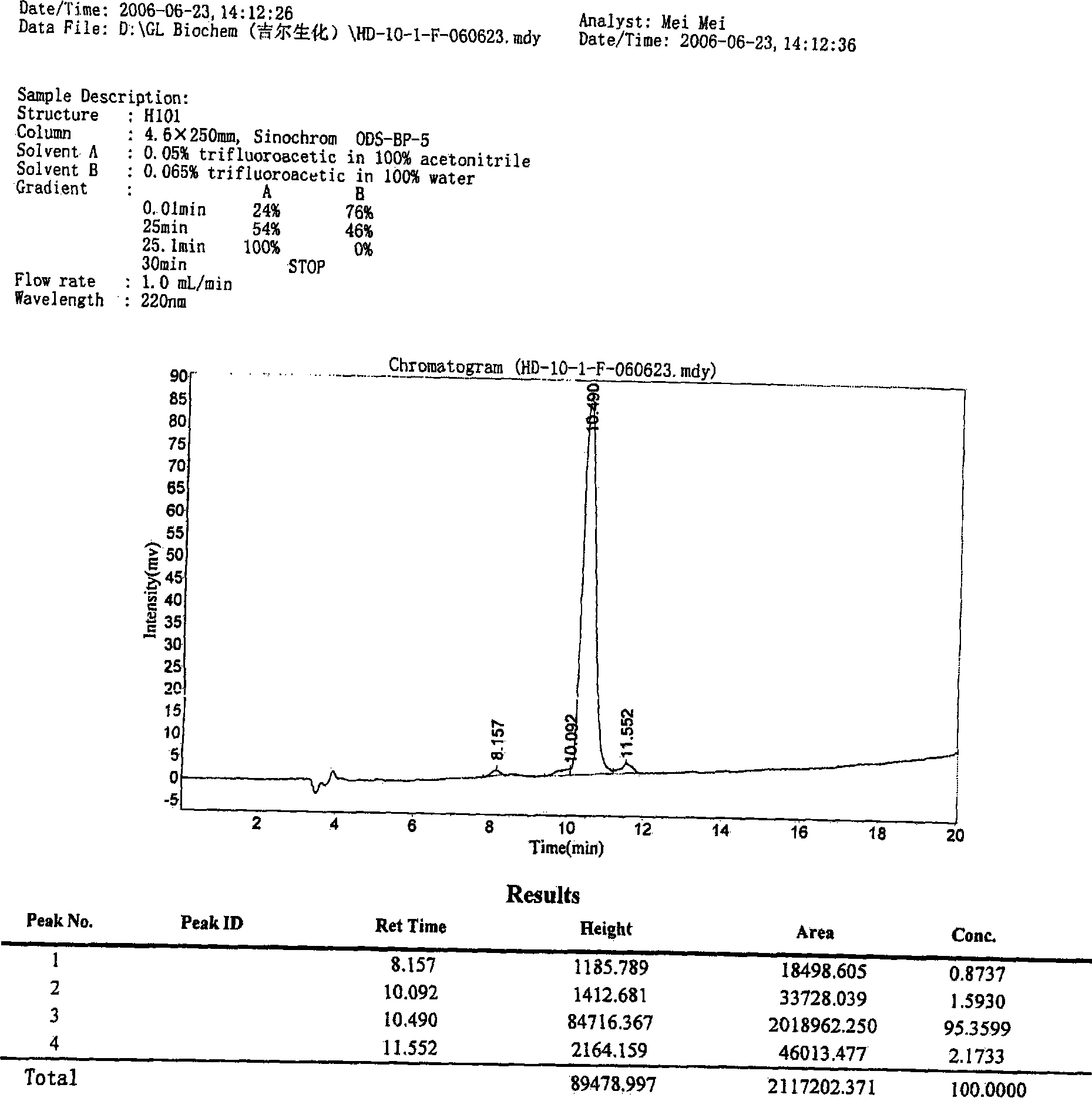
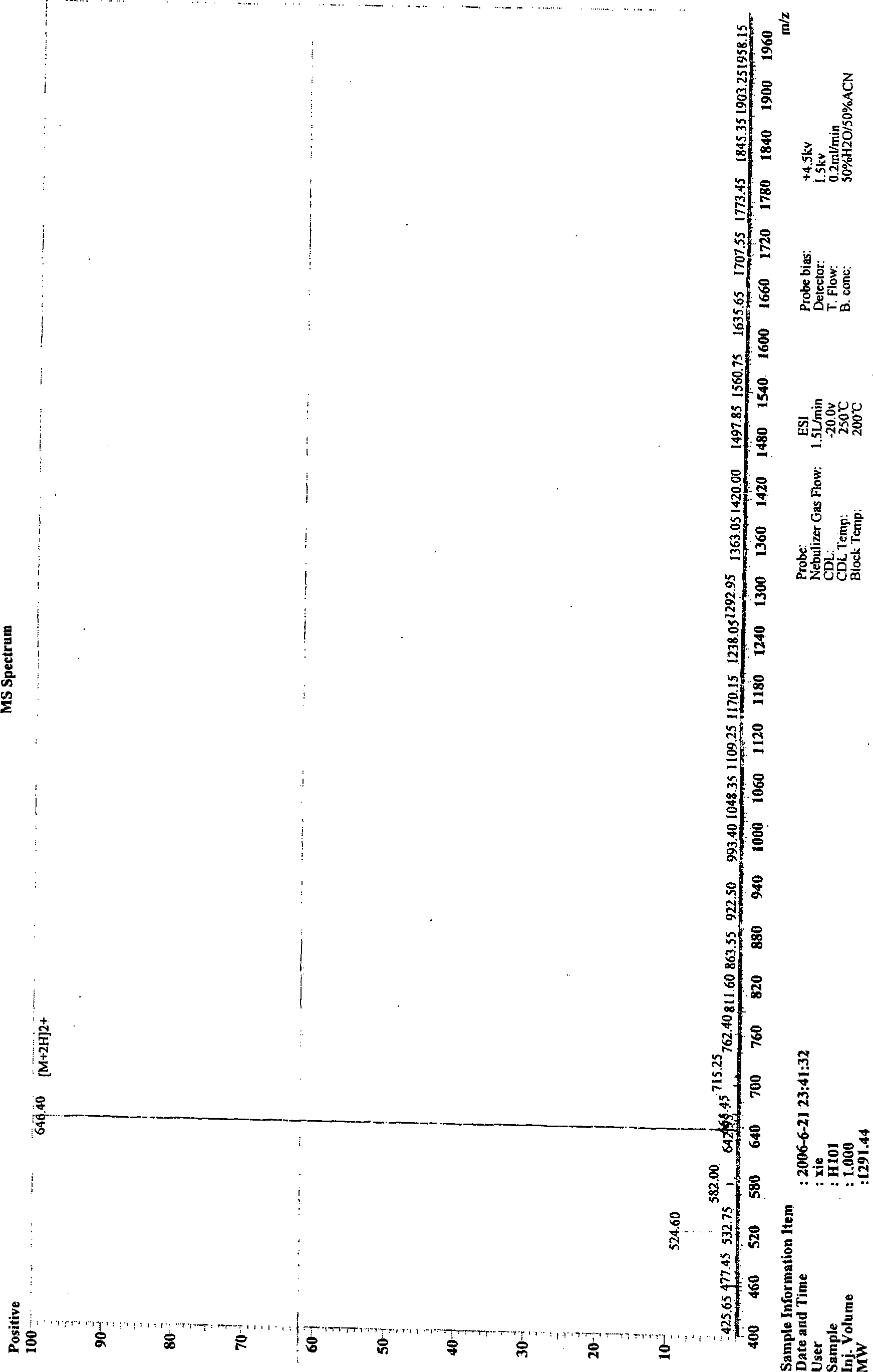
[0072] In one embodiment of the present invention, the polypeptide of the present invention is synthesized by Fmoc solid-phase synthesis method, and purified by HPLC. Fig. 1 shows in an exemplary manner the main steps of synthesizing and purifying a polypeptide H101 of the present invention and the detection results of the pr...
Embodiment 1
[0076] Embodiment 1: the preparation of polypeptide of the present invention
[0077] In this example, a polypeptide H101 of the present invention was first synthesized using the Fmoc / tBu solid-phase polypeptide synthesis method, and the sequence of the polypeptide is:
[0078] His-Lys-Gln-Leu-Val-Phe-Phe-Glu-Glu-Asp (SEQ ID NO: 1).
[0079] The raw materials used in the polypeptide synthesis method of this example are: Fmoc-Asp(OtBu)-Wang Resin(0.37mmol / g), Fmoc-Val-OH, Fmoc-Glu(OtBu)-OH, Fmoc-His(trt)- OH, Fmoc-Gln(trt)-OH, Fmoc-Leu-OH, Fmoc-Lys(Boc)-OH and Fmoc-Phe-OH.
[0080] The main steps of the polypeptide synthesis and purification method used in this example are as follows: Figure 1A shown.
[0081] Figure 1A The formulation of the TFA reagent used in the shown protocol is: [TFA:H 2 O: ethanedithiol: phenol, mixed with a volume ratio of 92.5:2.5:2.5:2.5].
[0082] The prepared peptide crude product is purified by HPLC, so that the purity of the peptide finall...
Embodiment 2
[0088] Embodiment 2: The effect of the polypeptide of the present invention on β-amyloid protein
[0089] Materials and Methods
[0090] 1. Drugs and reagents: Aβ 1-42(purity>98%), vitamin E, Thioflavin T (Thioflavin T, ThT), thiazolyl blue (MTT), dimethyl sulfoxide (DMSO), were all purchased from Sigma Company of the United States; MEM medium, fetal bovine serum ( FBS), trypsin (trypsin) are all purchased from U.S. Gibco-BRL company; Several polypeptides H101, H102 and H103 of the present invention are prepared by the method described in Example 1; Methods A pentapeptide Leu-Pro-Phe-Phe-Asp was synthesized, which had the same sequence as the β-sheet blocking peptide iAbeta5 invented by Soto-Jara et al., and was named L5 in the present invention. The L5 peptide will be used as a control in this example and the following examples. The above peptides were all synthesized by Shanghai Jill Biotechnology Co., Ltd., and purified by high performance liquid chromatography (High p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com