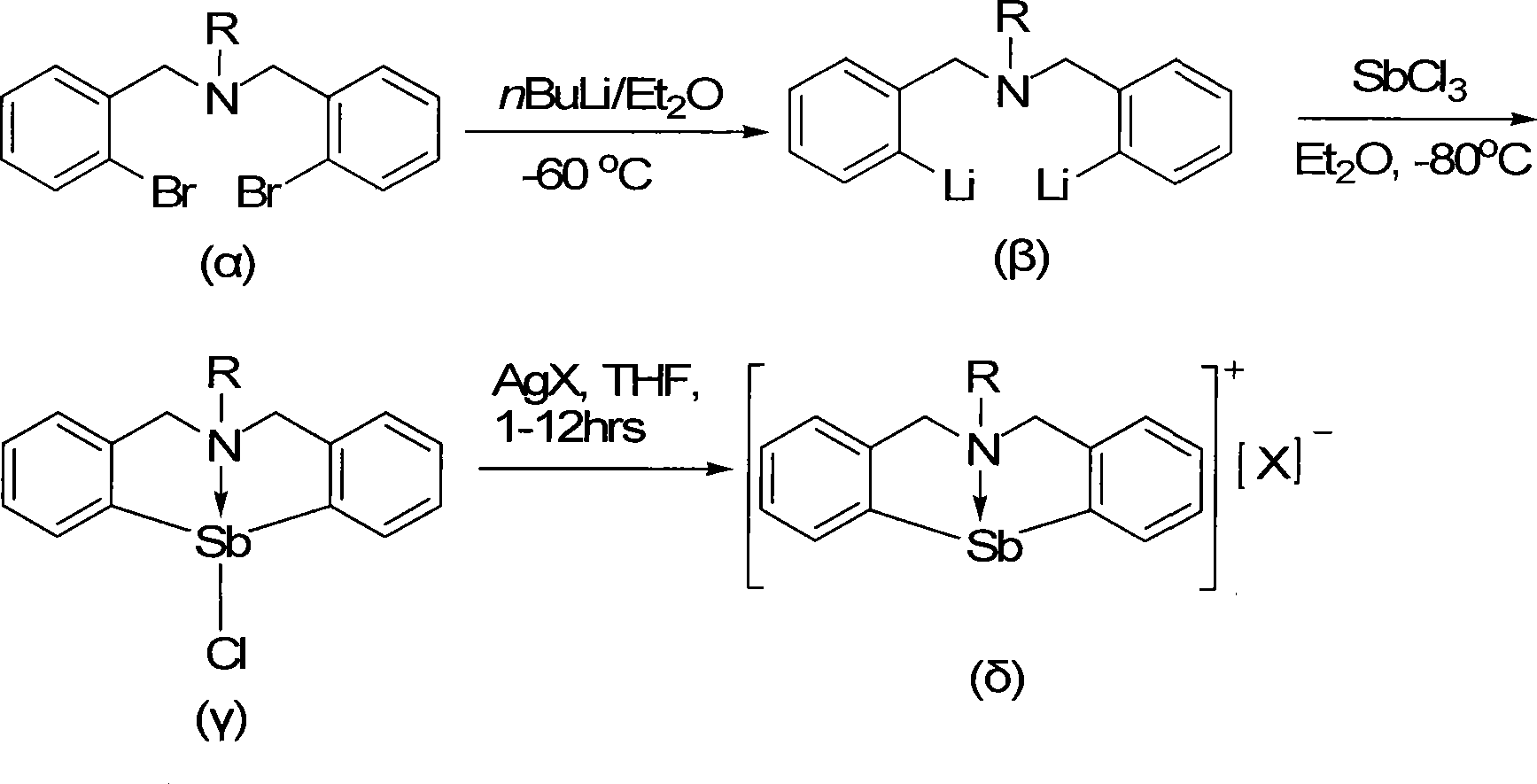
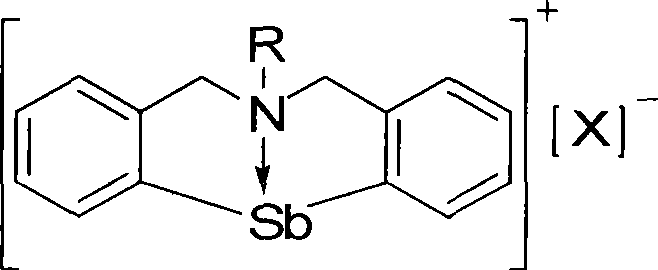
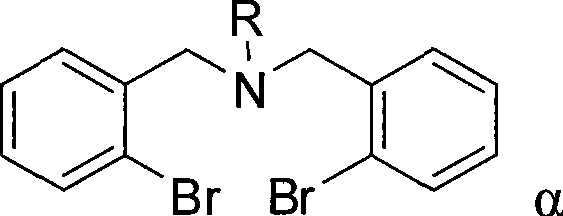
Organic antimony compound bearing a nitrogen-bridged ligand and its preparation and application
A technology of compound and antimony ion, which is applied in the field of organic antimony ion compound and its preparation, can solve the problems of poor stability of organic antimony compound, less application of organic antimony compound, weak Lewis acid, etc., achieve high catalytic activity and selectivity, and novel structure , prepare simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0034] In a 50mL one-necked flask, add 0.05mmol organic antimony ion compound (R=Ph, X=OSO 2 C 8 f 17) and 1.0 mmol benzaldehyde, after stirring for 5 minutes, tetraallyl tin (0.3 mmol) was added, placed in a water bath reactor with magnetic stirring, and reacted at room temperature 25° C. for 3 hours. TLC followed the reaction until the reaction was complete. The reaction result is: 1-phenyl-3-ene-butanol, the yield is 95.0%, and the selectivity of 1-phenyl-3-ene-butanol is 100%.
preparation example 2
[0036] In a 50mL one-necked flask, add 0.05mmol organic antimony ion compound (R=Cy, X=OSO 2 C 8 f 17 ) and 1.0 mmol benzaldehyde, after stirring for 5 minutes, tetraallyl tin (0.3 mmol) was added, placed in a water bath reactor with magnetic stirring, and reacted at room temperature 25° C. for 3 hours. TLC followed the reaction until the reaction was complete. The reaction result is: 1-phenyl-3-ene-butanol, the yield is 96.0%, and the selectivity of 1-phenyl-3-ene-butanol is 100%.
preparation example 3
[0038] In a 50mL one-necked flask, add 0.05mmol organic antimony ion compound (R=t-Bu, X=OSO 2 C 8 f 17 ) and 1.0 mmol benzaldehyde, after stirring for 5 minutes, tetraallyl tin (0.3 mmol) was added, placed in a water bath reactor with magnetic stirring, and reacted at room temperature 25° C. for 3 hours. TLC followed the reaction until the reaction was complete. The reaction result is: 1-phenyl-3-ene-butanol, the yield is 98.0%, and the selectivity of 1-phenyl-3-ene-butanol is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com