H5 subtype bird-flue virus H5N1 hemagglutinin monoclonal antibody and nucleic acid sequence and preparation method thereof
A technology of monoclonal antibody and avian influenza virus, which is applied in the preparation of the above-mentioned antibody, the variable region gene of the above-mentioned antibody, and the field of highly neutralizing active anti-H5 subtype avian influenza virus hemagglutinin-specific monoclonal antibody, which can solve the problem of Problems such as low blood inhibitory titer and unknown H5N1 virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] The antibody of the present invention will be further illustrated by specific examples and experimental reports below:
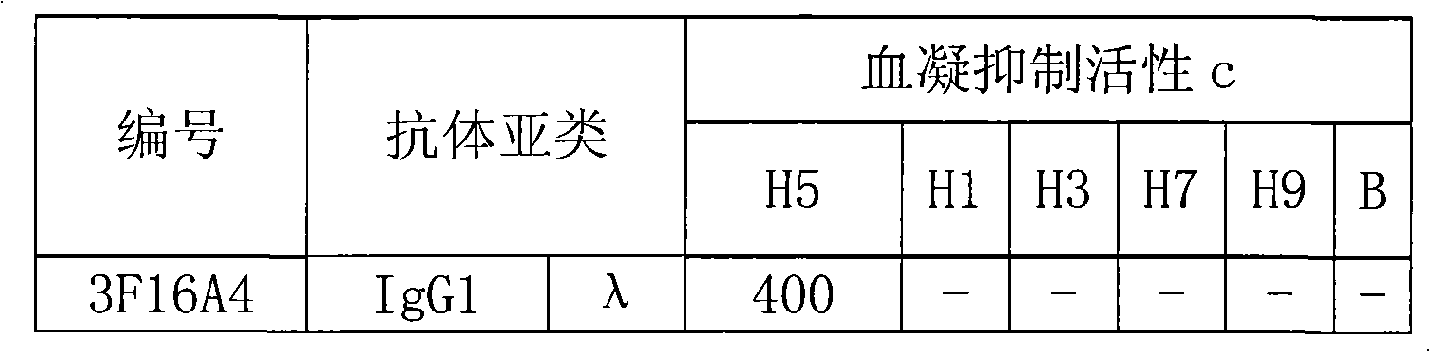
[0016] 1. Preparation of H5 Subtype Avian Influenza Virus (H5N1) Hemagglutinin Monoclonal Antibody
[0017] 1.1 Preparation of immunogen
[0018] The natural H5 hemagglutinin (H5N1 A / Goose / Guangdong / 1 / 96) was used as the immunogen, and the H5 hemagglutinin (A / Goose / Guangdong / 1 / 96) was obtained from Harbin Veterinary Research Institute. The titer was detected by hemagglutination test to be 1:1024. (The specific methods of hemagglutination test and hemagglutination inhibition test refer to the WHO operation guide)
[0019] 1.2 Immunization of mice
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com