Stable and ready-to-use oil-in-water propofol microemulsion
A technology of microemulsion and propofol, which is applied in the direction of emulsion delivery, medical preparations of non-active ingredients, liquid delivery, etc., can solve the problems of side effects, the importance of not clearly increasing the concentration of propofol, etc., and achieve effective hypnosis and Lower risk of anesthesia, side effects and potential adverse effects, safer and more reliable short-term or long-term effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
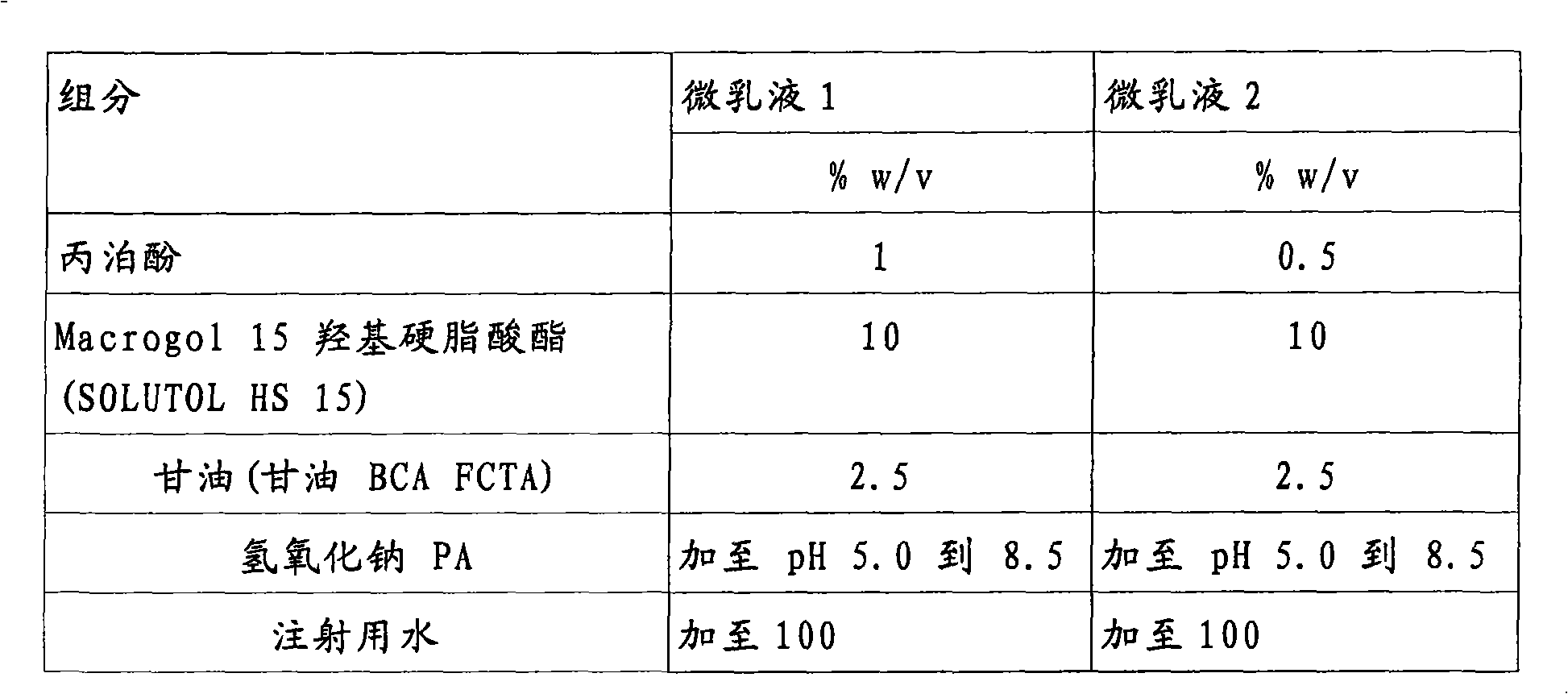
[0050] Preparation of embodiment 1.1% and 0.5% propofol-containing microemulsions.
[0051] A complete formulation of the present invention can be described as Microemulsion 1 and Microemulsion 2 formulations, the ratios of which are preferred propofol and excipients are described in Table 1 as follows:
[0052] Table 1: Propofol microemulsions.
[0053]
[0054] According to the present invention, microemulsion 1 and microemulsion 2 are prepared as follows:
[0055] 70% of the total water for injection was added to a stainless steel reactor with a stirring system. In addition, macrogol 15 hydroxystearate (SOLUTOL HS 15) was added into a stainless steel container, and heated to 50°C with constant stirring to obtain a completely melted product. Next, 6% of the total water for injection and propofol were added to the melted surfactant with constant stirring.
[0056] The contents of the stainless steel vessel were added to a reactor filled with 70% total water for injectio...
Embodiment 2
[0060] Example 2. The stability of propofol microemulsion of the present invention.
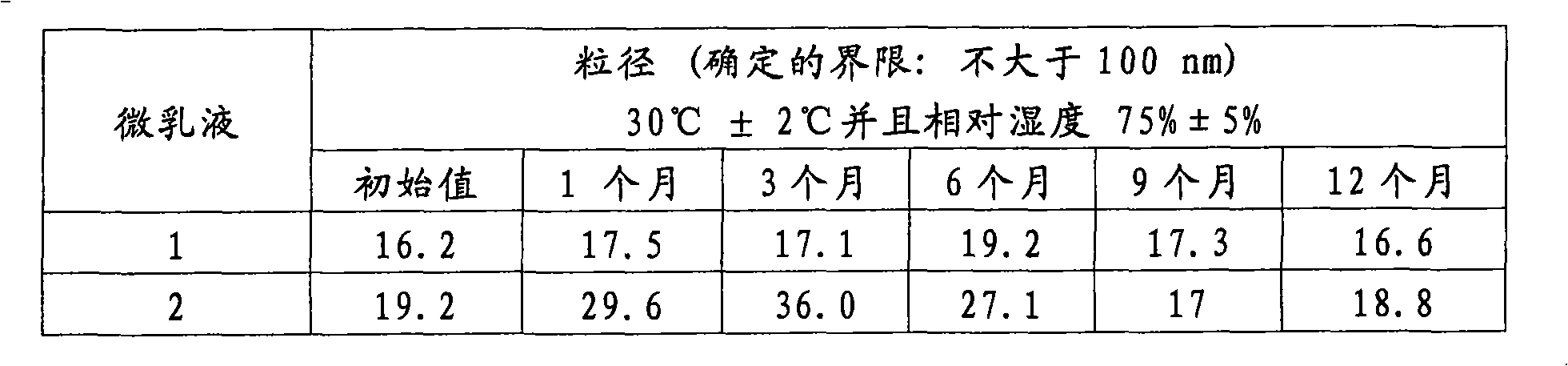
[0061] Freshly prepared microemulsions 1 and 2 according to Example 1 were tested to evaluate their properties. Evaluation of the particle size is the most important issue of the present invention, since the stability of this parameter is a drawback of the propofol microemulsions described in the prior art.
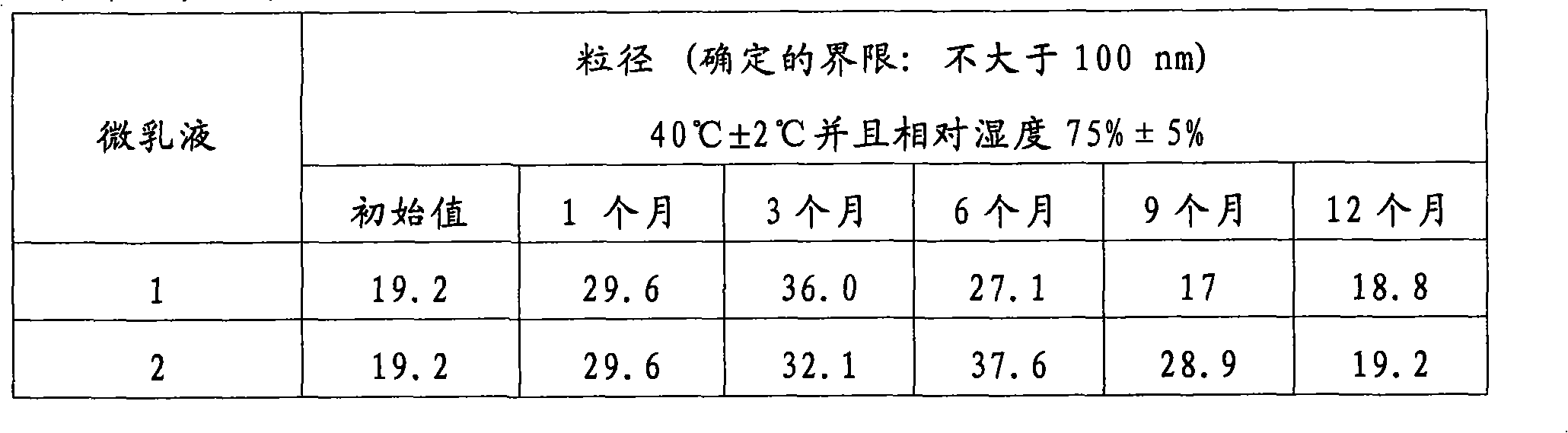
[0062] Particle size was monitored during a 12 month stability test (see Table 2) under normal temperature conditions and a 180 day accelerated stability test at 40°C (Table 3).
[0063] The results shown in Tables 2 and 3 show that there was not any significant change in particle size during the monitoring time. Even earlier samples exhibit particle sizes significantly smaller than the maximum 50nm maximum established by the present invention as the preferred limit.
[0064] Table 2. Effect of storage time on the particle size of propofol microemulsions according to the usual conditio...
Embodiment 3
[0068] Example 3. Comparative analysis of the stability of the propofol microemulsion of the prior art and the microemulsion of the present invention.
[0069] The formulations are described in the referenced patent US 6,743,436 (Examples 1, 5 and 6), which were chosen for comparison to illustrate the improvement of physicochemical properties achieved by the pharmaceutical composition of the present invention, as the closest existing comparison to this new composition. have technology.
[0070] When the composition was again prepared as taught by the referenced patent, it was observed that the general turbidity of the composition did not comply with the parameters of a microemulsion, and the analysis showed a particle size well above the limit of 100 nm (see Table 4).
[0071] Table 4. Determination of particle size in accelerated stability test (40°C ± 2°C).
[0072]
[0073] * Poloxamer is a surfactant, combined with Solutol HS15 as a co-surfactant;
[0074] ** Poloxame...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com