Bioabsorbable medical device with coating
A medical device, bioabsorption technology, applied in coating, medical science, surgery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
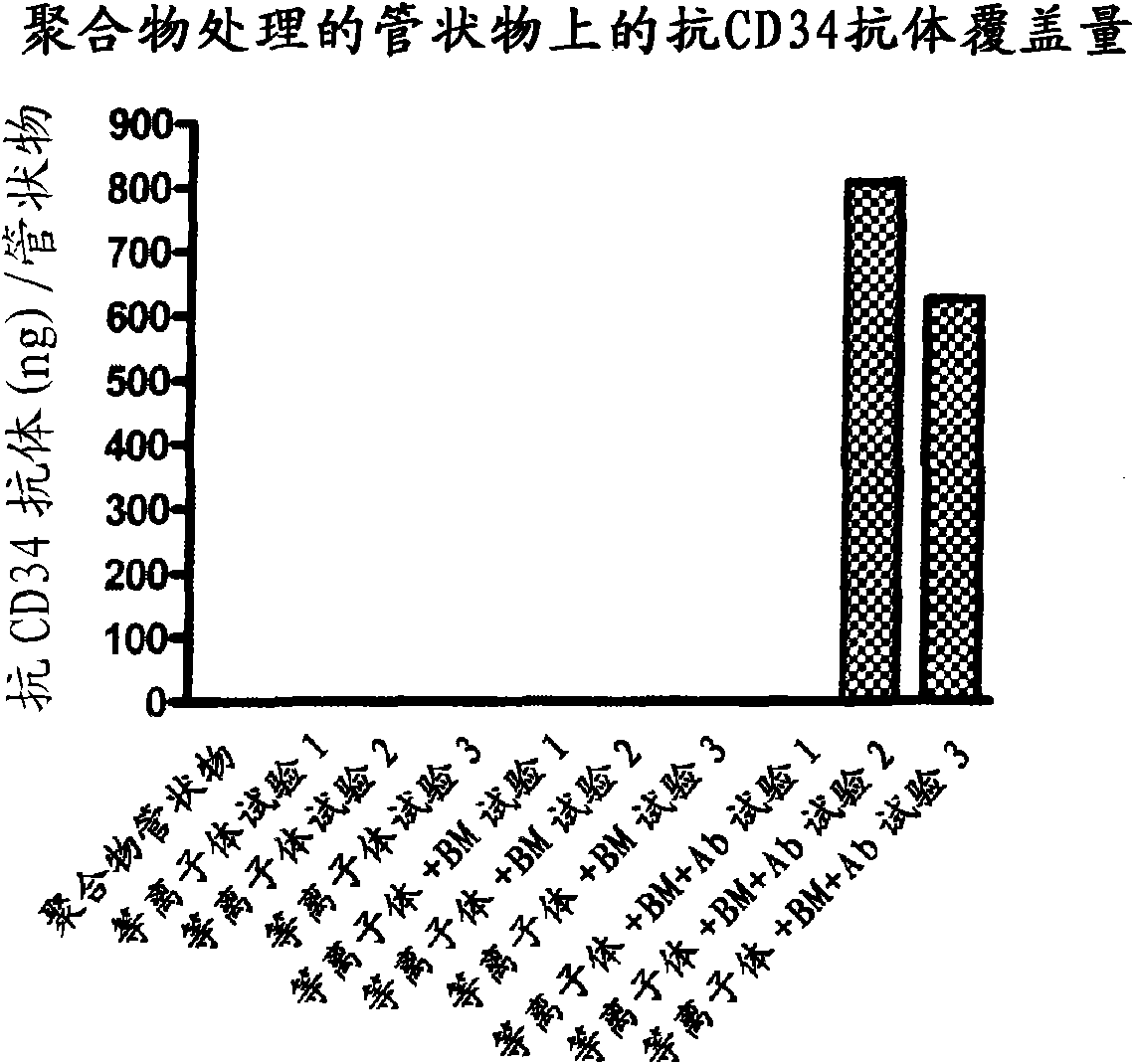
[0131] Bioabsorbable polymeric tubing used in the preparation of embodiments of the present invention is prepared from the polymer compositions described above. Uncoated tubing as well as tubing that had been coated as described above (comprising a coating with anti-CD34 antibody coated on a polymer matrix) were analyzed for their ability to bind antibodies to their surface. Prior to testing the tubing for cell binding, the antibody-coated tubing was checked for the amount of antibody bound to its surface. The experiment was repeated 3 times. The results of these studies are shown in figure 2 middle.
[0132] Such as figure 2 As shown in , the untreated tubing, the plasma treated (oxygen plasma followed by argon plasma) tubing, and the matrix-coated tubing did not each fail in any of the experiments performed. Contains any anti-CD34 antibody. In contrast, polymer tubes coated first with a coating solution comprising a bioabsorbable polymer and then with a buffer solutio...
Embodiment 2
[0134] The non-coated and coated tubing as well as the plasma-treated tubing were incubated with Kg1a cells. Following incubation, the tubing was rinsed in buffered saline to remove unbound cells, and the tubing was fixed and processed to identify cells bound to the device. Tested tubules were stained by fluorescent (nucleostain DAPI ((4',6-diamidino-2-phenylindole) dihydrochloride)) staining after incubation and examined under a fluorescent microscope Binding of cells to the tubing was determined. The results of these experiments are shown in Figure 3-6 .
[0135] image 3 A representative bioabsorbable tube is shown with a coating comprising polymer and anti-CD34 antibody, depicting the adhesion of many Kg1a cells to the tube, which can be visualized by the fluorescence emitted from the cells. Figure 4 is a representative non-coated tubing from the experiment showing few adherent cells on the non-coated tubing. Most of the signal from these panels appeared to be due t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com