Method for marking bifluorescence protein molecule cell
A labeling method and dual-fluorescence technology, which can be applied to cells modified by introducing foreign genetic material, introducing foreign genetic material using a vector, recombinant DNA technology, etc. It can solve the problems of labeling that are not suitable for living cells, and save the time of labeling. , the effect of simple marking process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 , Construction of recombinant plasmids
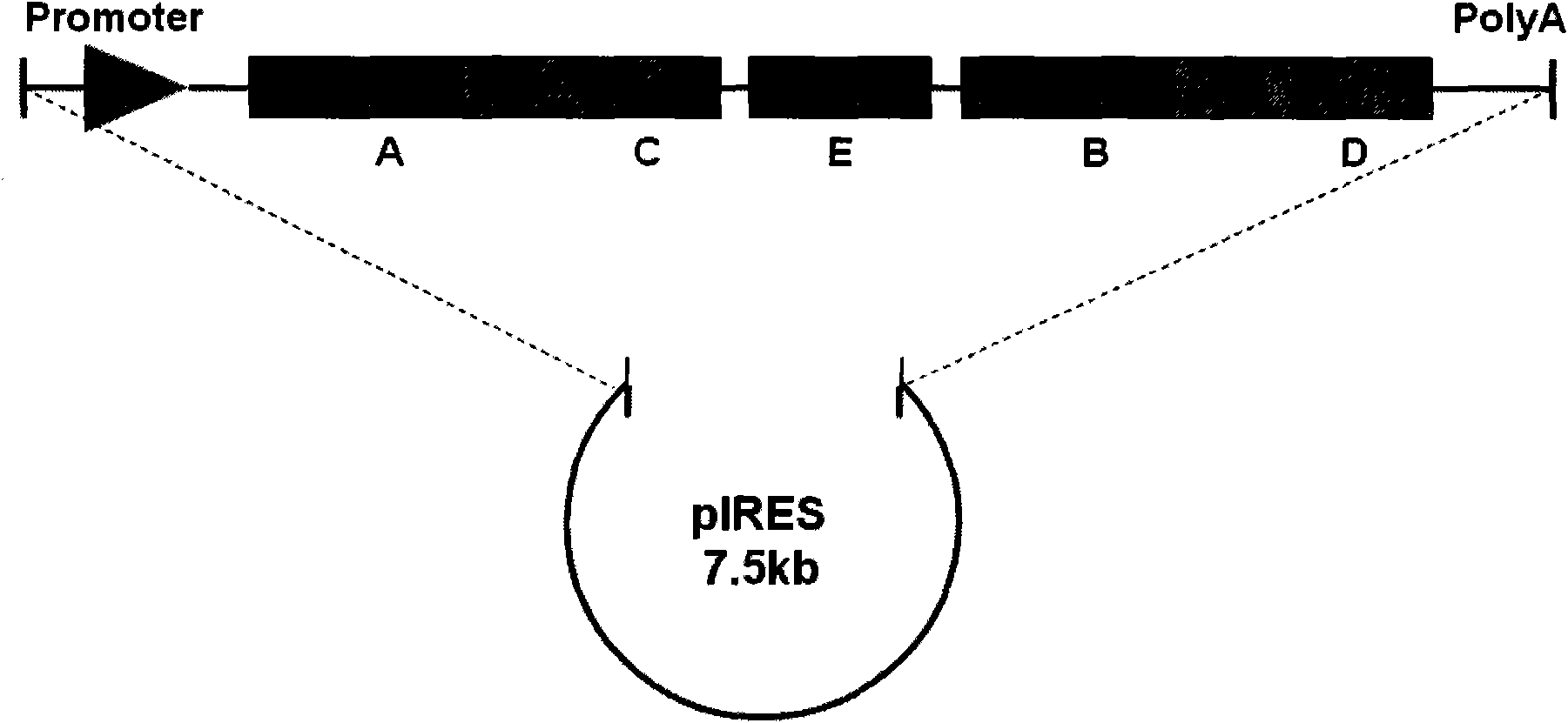
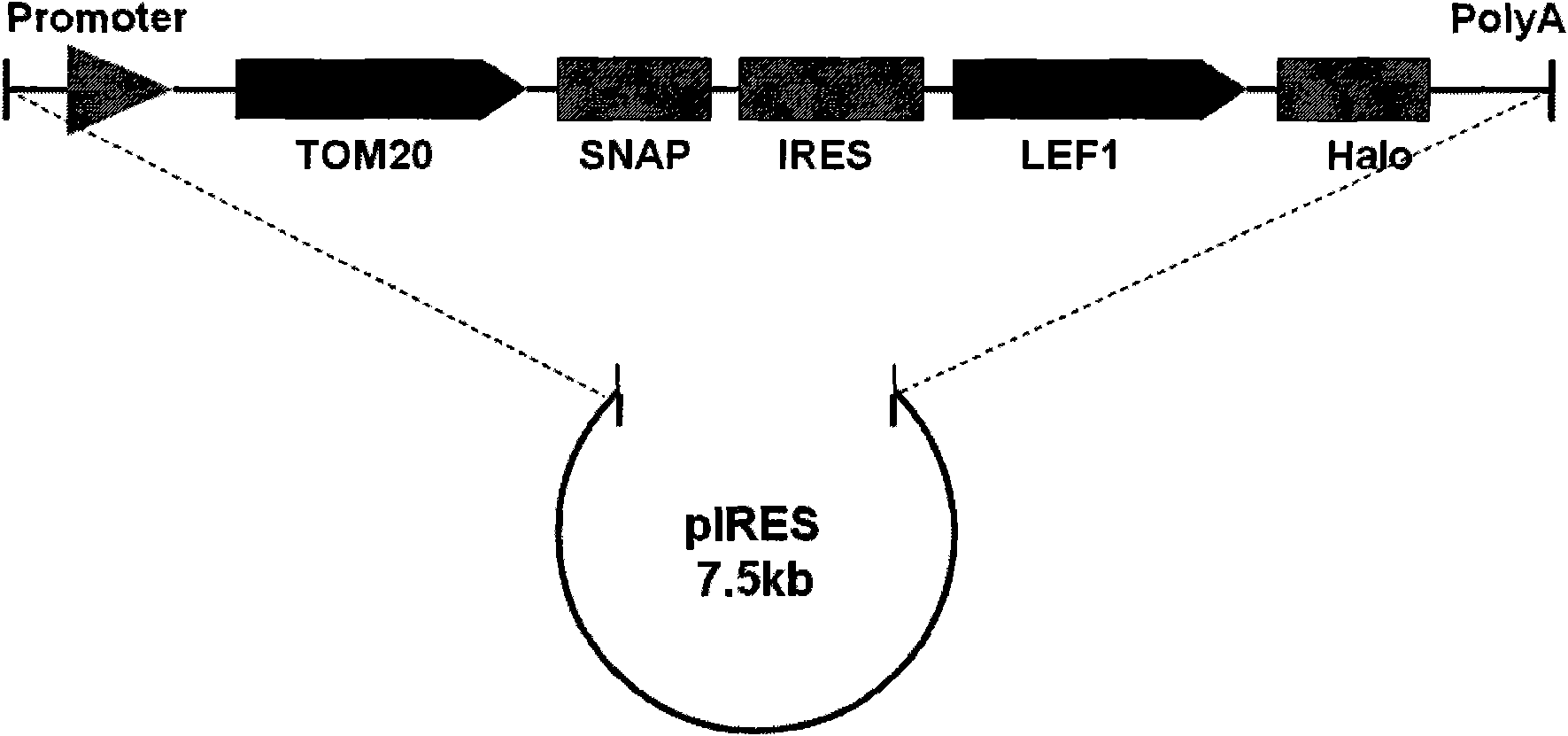
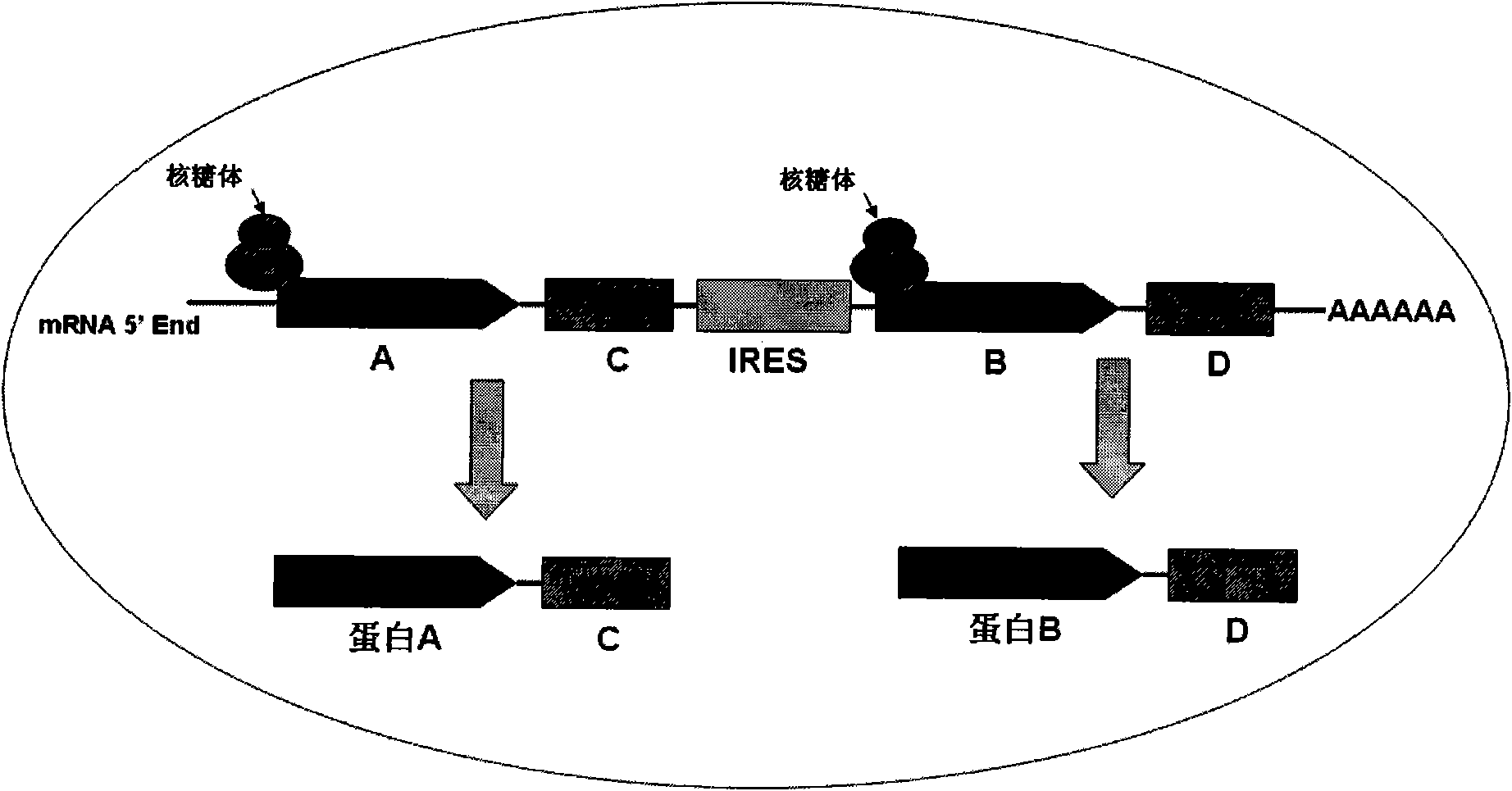
[0020] In this example, the inventor constructed the pTOM20-SNAP-IRES-LEF1-Halo recombinant plasmid, in which the inventor cloned the pTOM20-SNAP-IRES-LEF1-Halo fragment, wherein the protein LEF1 is the Wnt signal The protein molecules in the pathway are mainly located in the nucleus. The protein TOM20 is a protein on the outer membrane of the mitochondria, which is located on the mitochondria; IRES is a bicistronic ORF expression system of the internal ribosome insertion site element, making the two genes Two ORFs are co-expressed under the same promoter in one vector, the first cistron expression cassette is fused with SNAP at the C-terminus, and the second is fused to express Halo.
[0021] The construction of the pTOM20-SNAP-IRES-LEF1-Halo fragment includes the following 4 steps: 1. Connect the LEF1 obtained by PCR into the pFC14A vector with Halo to obtain the pFC14A-LEF1 recombinant plasmid; 2. Digest the LEF1- ...
Embodiment 2
[0052] Example 2 , transfected cells and fluorescent labeling
[0053] 2.1. Cell transfection
[0054] Use the Lipo-plus method to transfect Hela cells, the specific steps are as follows:
[0055] (1) Put the slides cleaned and sterilized with medical alcohol into a 24-well plate, coat them with poly-L-Lysine (Poly-L-Lysine) for 30 minutes, wash them with sterilized water for 3-5 times, and wash away Uncoated PLL, dry on a clean bench;
[0056] (2) Hela cells were cultured for 24 hours in a 24-well plate coated with PLL slides;
[0057] (3) Take 250ng of pTOM20-SNAP-IRES-LEF1-Halo recombinant plasmid and add it to the EP tube containing 25ul serum-free DMEM culture medium, and mix well;
[0058] (4) Take the transfection reagent plus, dilute it into DMEM culture medium at 1:20, mix well, add 21ul / tube into the EP tube in the previous step, mix well, and let stand for 15 minutes;
[0059] (5) Take the transfection reagent lipo, dilute it into DMEM culture medium at 1:20, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com