Inhibition of degradation of extracellular matrix
An extracellular matrix and cell technology, applied in the field of heparanase inhibitors, can solve problems such as islet function loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
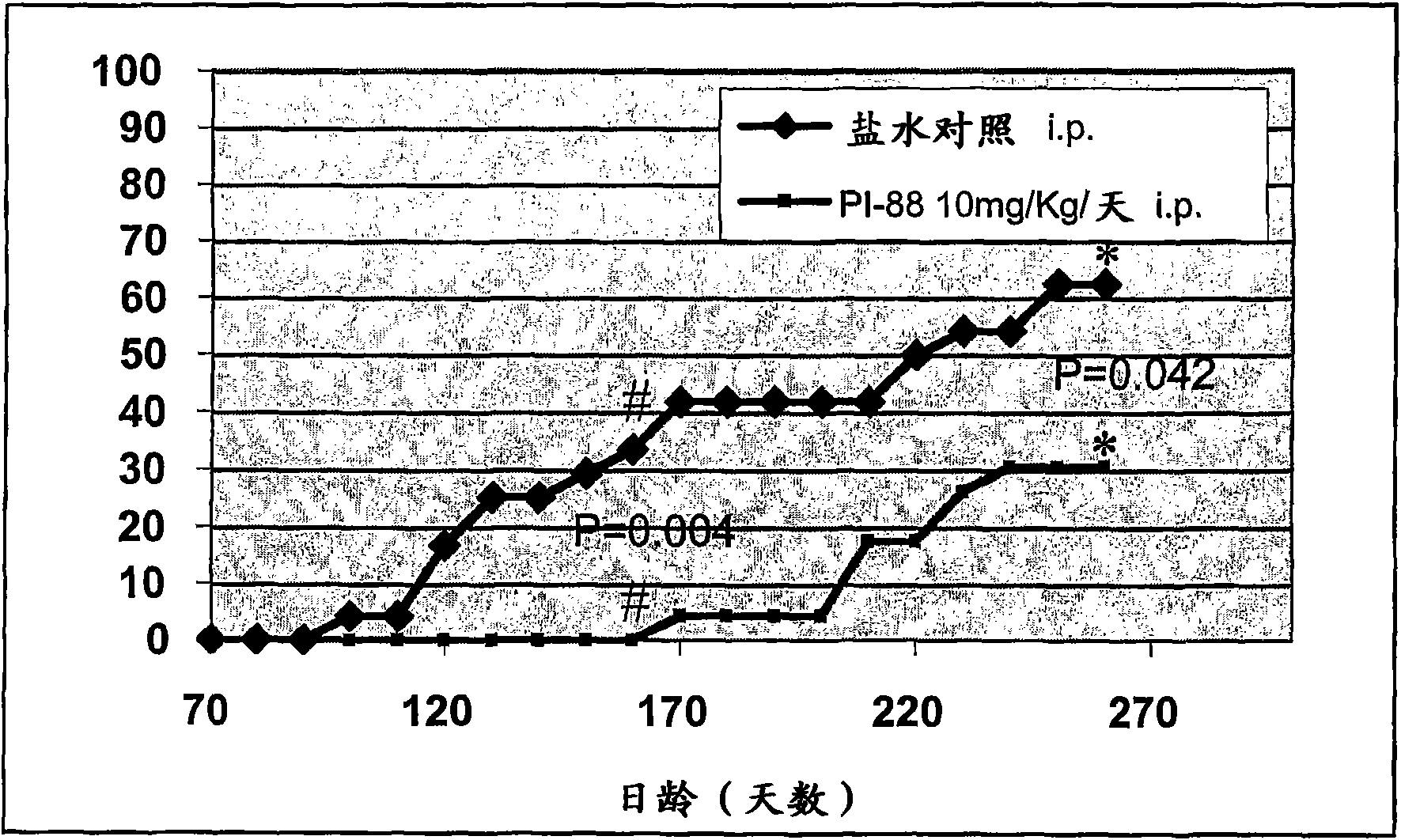
[0173] The role of heparanase in triggering destructive insulitis and clinical diabetes
[0174] Studies performed by the present inventors have shown a 7-fold increase in heparanase transcripts in pre-diabetic and diabetic-onset NOD mice compared to neonatal NOD mice; only background levels were detected in normal CBA / H mice (See Table 1). These results have been strengthened by evidence that treatment of 10-11 week old female NOD / Lt mice with the heparanase inhibitor PI-88(9) prevents clinical diabetes in mice up to 24 weeks of age onset (see image 3 ). Furthermore, the inventors have found that delivery of purified human platelet-derived heparanase via the pancreatic duct into conventional (non-autoimmune) mice results in in situ destruction of islet BM and intraislet HS ( See Figure 4 ). These results are consistent with a role for heparanase in triggering destructive insulitis in NOD mice and demonstrate the ability of PI-88 to protect NOD mice from onset of clinic...
Embodiment 2
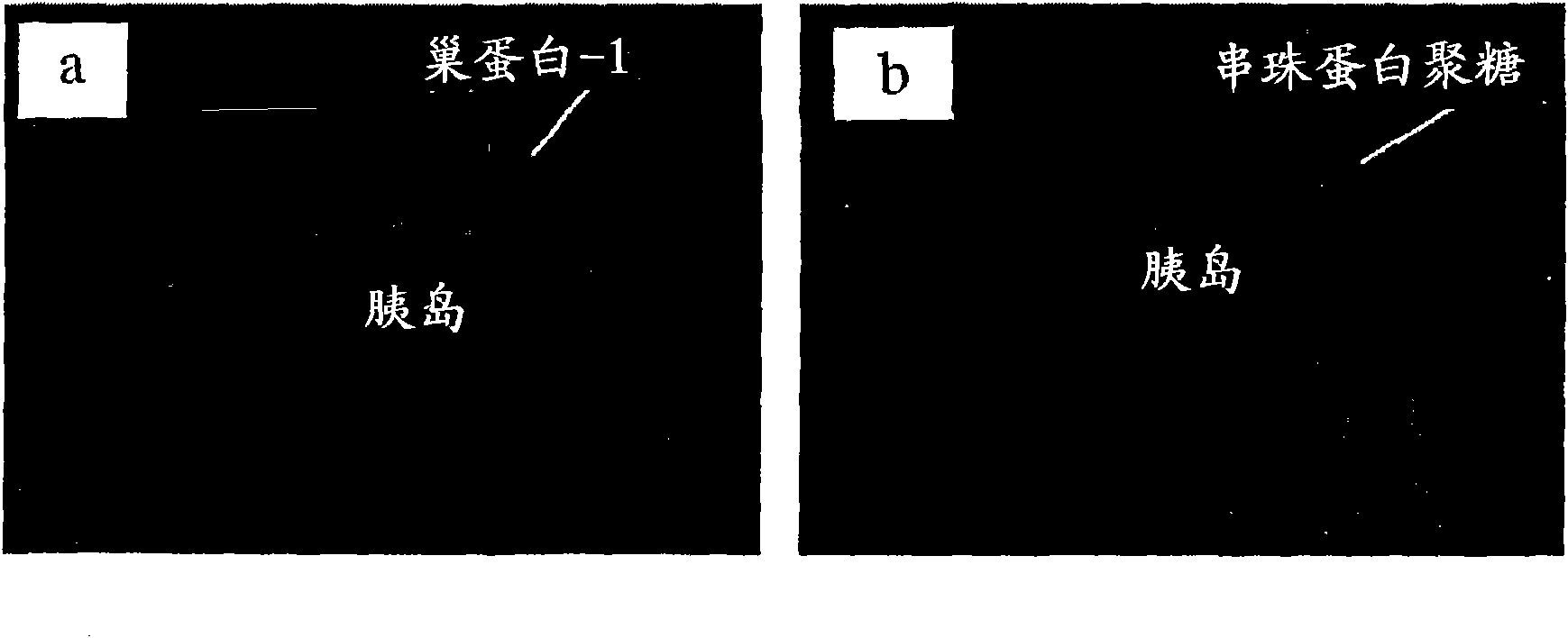
[0179] Intragraft expression kinetics of heparanase mRNA in islet isografts undergoing autoimmune destruction in diabetic NOD mice
[0180] In general, histopathology of pancreas from prediabetic female NOD and autoimmune destruction of syngeneic islets transplanted into diabetic NOD mice showed that autoreactive T cells and other MNCs did not enter islets via intraislet blood vessels . Instead, invasive leukemia moves from the periislet location into the islet cell mass after disintegration of the islet BM by degradative enzymes such as heparanase produced locally at the islet BM interface by activated leukocytes. Thereafter, the process of MNC infiltration can lead to the degradation of ECM heparan sulfate in the islets and the destruction of the islets.
[0181] Investigations into whether this enzyme-dependent leukocyte invasion mechanism is functional in the autoimmune destruction of islet isografts in diabetic NOD mice include general experiments such as examining islet...
Embodiment 3
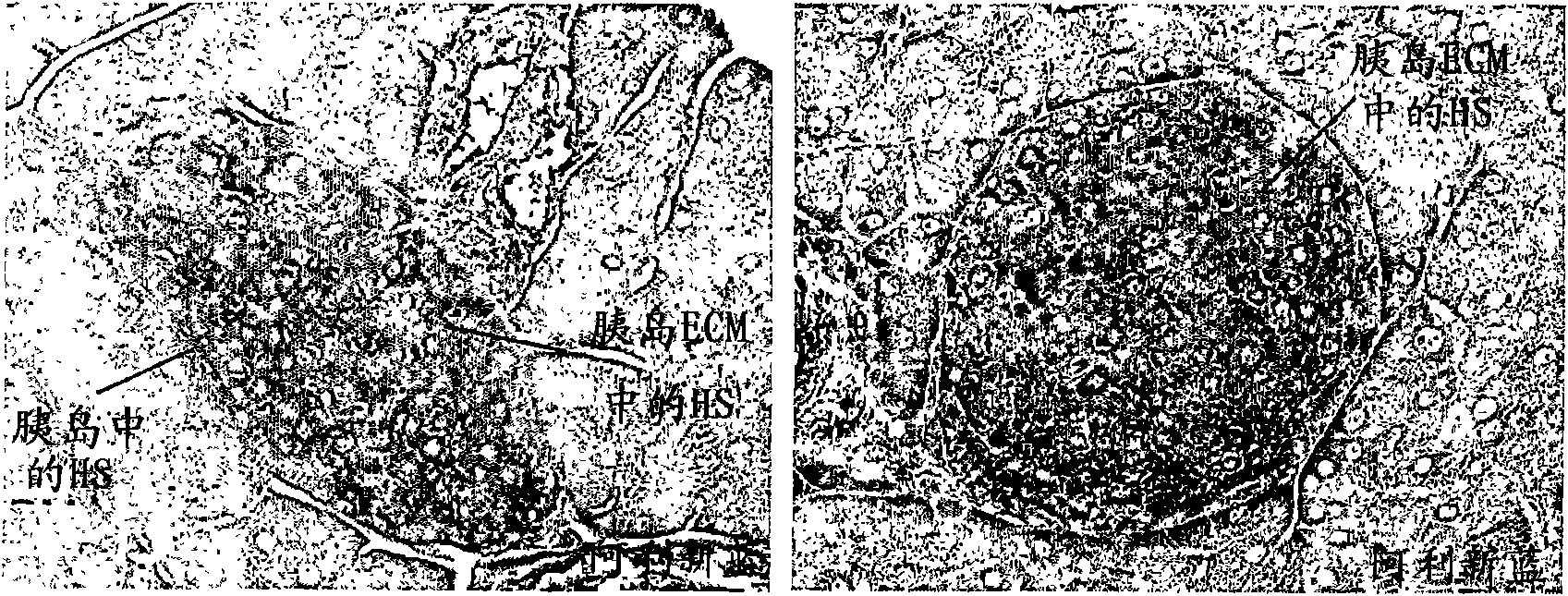
[0190] In situ expression of heparan sulfate (HS) in NOD islets and degradation of HS in islets during destructive insulitis
[0191]In addition to the trechanin / HS present in the islet BM, HSPG is also a component of the more widely distributed ECM. The ECM consists of a network of macromolecules whose function is to fill the extracellular space in tissues and provide tissue-specific cells with a scaffold on which invading leukocytes can migrate (3). Indeed, it has been shown that the survival and function of β cells depend on the maintenance of their interaction with the intraislet ECM (23, 24). Therefore, heparanase may not only facilitate the entry of activated MNCs through the islet BM, but also degrade intraislet ECM, thereby not only promoting the migration of invading MNCs but also reducing the viability of adjacent β cells.
[0192] General experiments to confirm the relationship of islet-associated heparan sulfate and heparanase to islet integrity included not only ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com