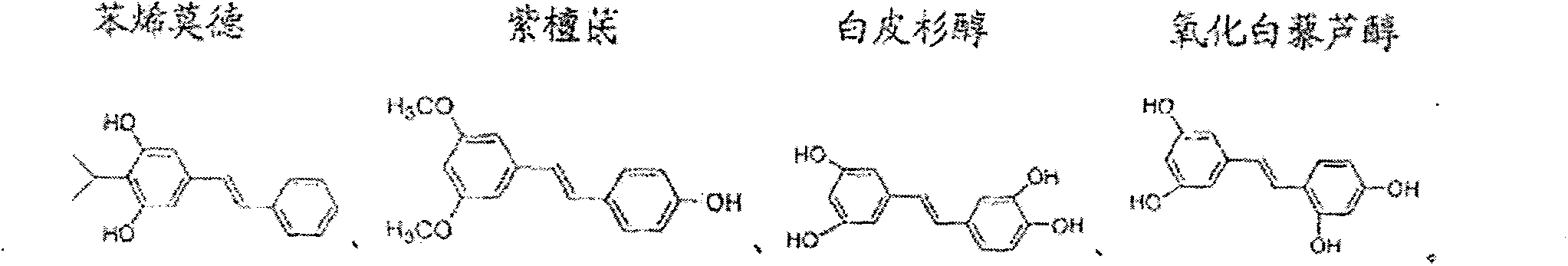
Method for synthesizing stilbenoids by hydrochloric acid heterogeneous chlorination
A heterogeneous, hydrochloric acid technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as difficult separation, high impurity content, and pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11-15
[0047] Embodiment 11-15 Method 2 for the heterogeneous chlorination of hydrochloric acid to synthesize piceatanol
[0048]
[0049]
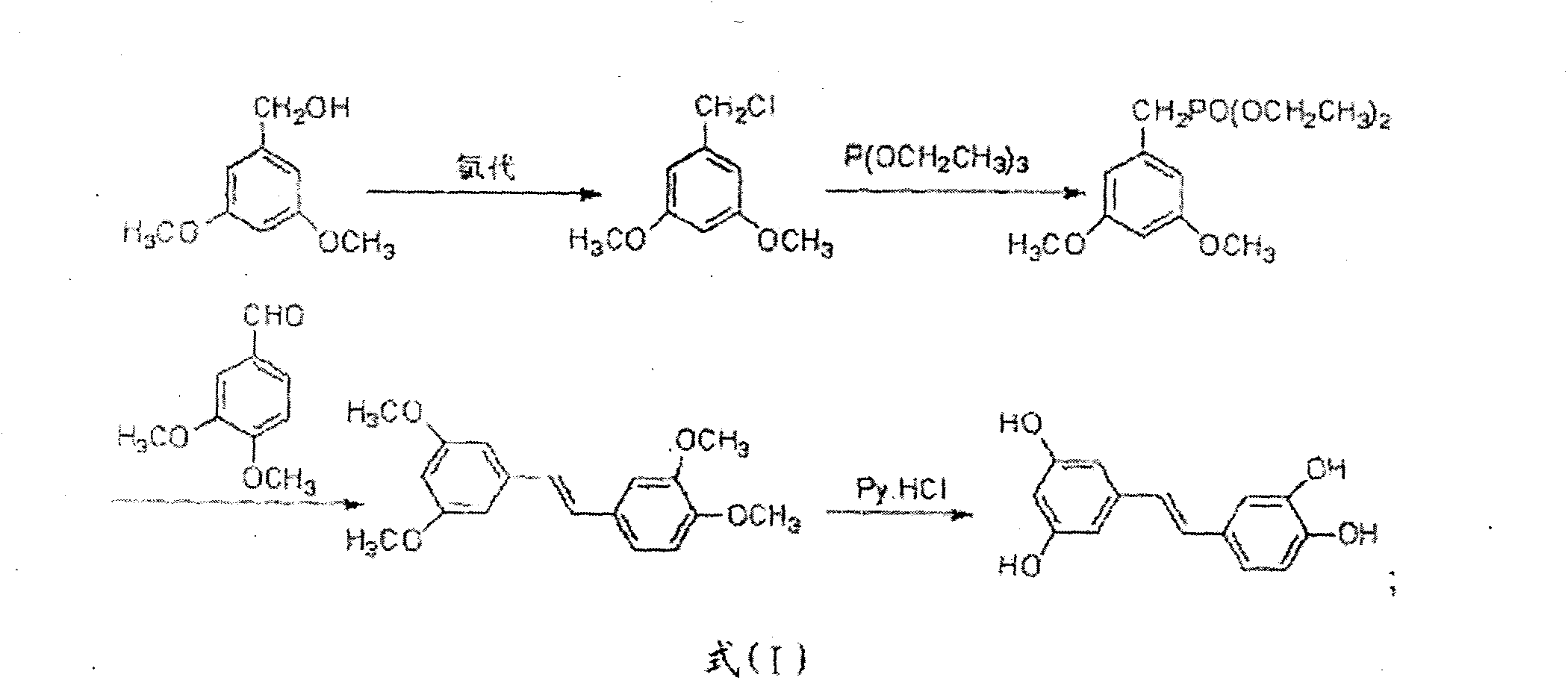
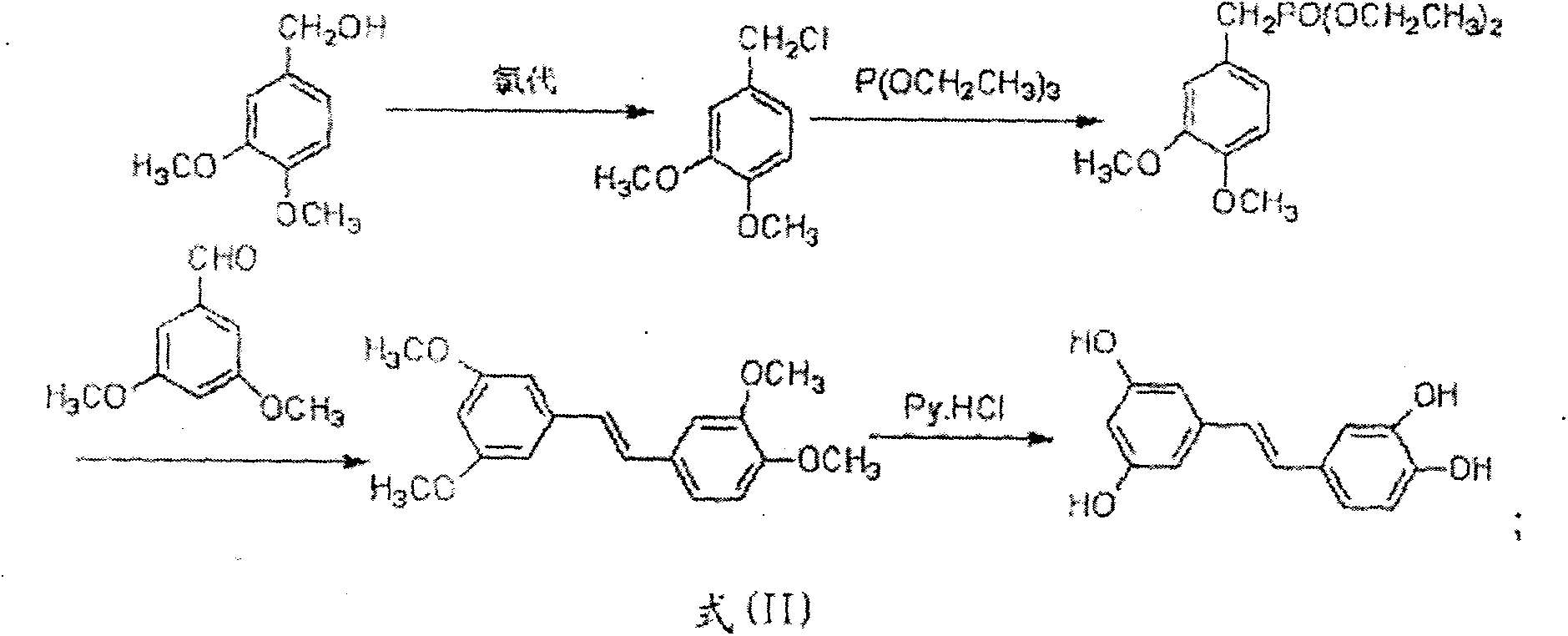
[0050] The method two of above-mentioned heterogeneous chlorination of hydrochloric acid to synthesize piceatanol, the reaction scheme is as formula (II):
[0051]
[0052] The method two of the above-mentioned heterogeneous chlorination of hydrochloric acid to synthesize piceatanol is carried out according to the following steps:
[0053] (41) Preparation A4: 3,4-dimethoxybenzyl chloride
[0054] Take 3,4-dimethoxybenzyl alcohol, add solvent, and slowly drop hydrochloric acid; after the reaction is completed, let stand to separate layers, wash the organic phase, dry, and evaporate the organic solvent to obtain product A4;
[0055] (42) Preparation B4: Diethyl 3,4-dimethoxybenzylphosphonate
[0056] A4 is mixed with triethyl phosphite, heated, refluxed, monitored by TLC, and processed after the reaction is completed to obtain B4;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com