Heterogenetic antigen-Fc fusion protein capable of inducing antitumor immunity of organism and application thereof
A technology of fusion protein and anti-tumor activity, applied in the field of heterologous antigen-Fc fusion protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. The immunomodulatory effect of fusion protein neu-Fc combined with GM-CSF and BCG
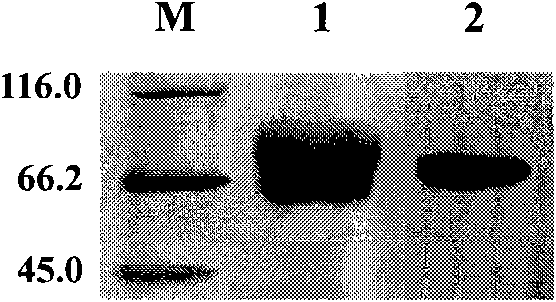
[0039] 1. Construction, expression and purification of neu-Fc
[0040] The extracellular domain of rat neu includes four domains, L1, S1, L2 and S2. The primers 5'-tgccatggtccgagtgtgctatggtctg-3' and 5'-agtttagcggccgcgtgggtgcagttgatggg-3' were designed according to the L2 and S2 domains of the extracellular domain of rat neu, and the coding sequences of the L2 and S2 domains of the extracellular domain of rat neu were amplified by PCR ( The upstream primer is located in the L2 domain of rat neu extracellular domain, and the downstream primer is located in the S2 domain of rat neu extracellular domain).
[0041] The total RNA of isolated peripheral blood mononuclear cells of healthy people was extracted, and the cDNA of human IgG1Fc segment was amplified by RT-PCR method. The cDNA of human IgG1 Fc segment was digested with NotI and XhoI and inserted into the vector pCI-neo (purchase...
Embodiment 2
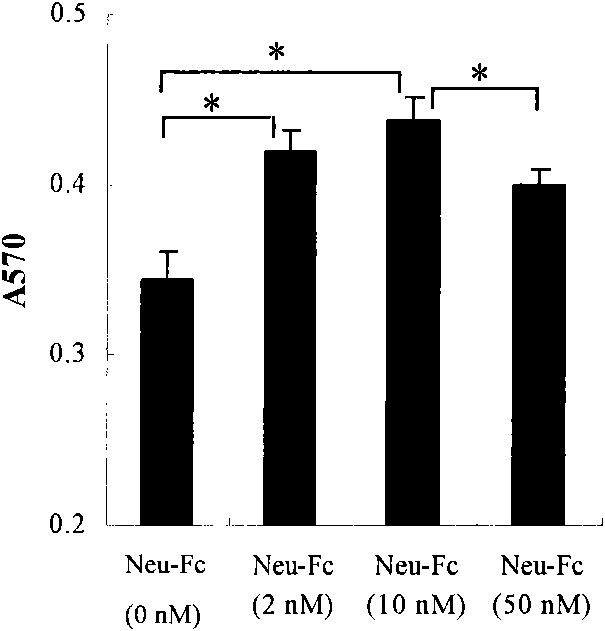
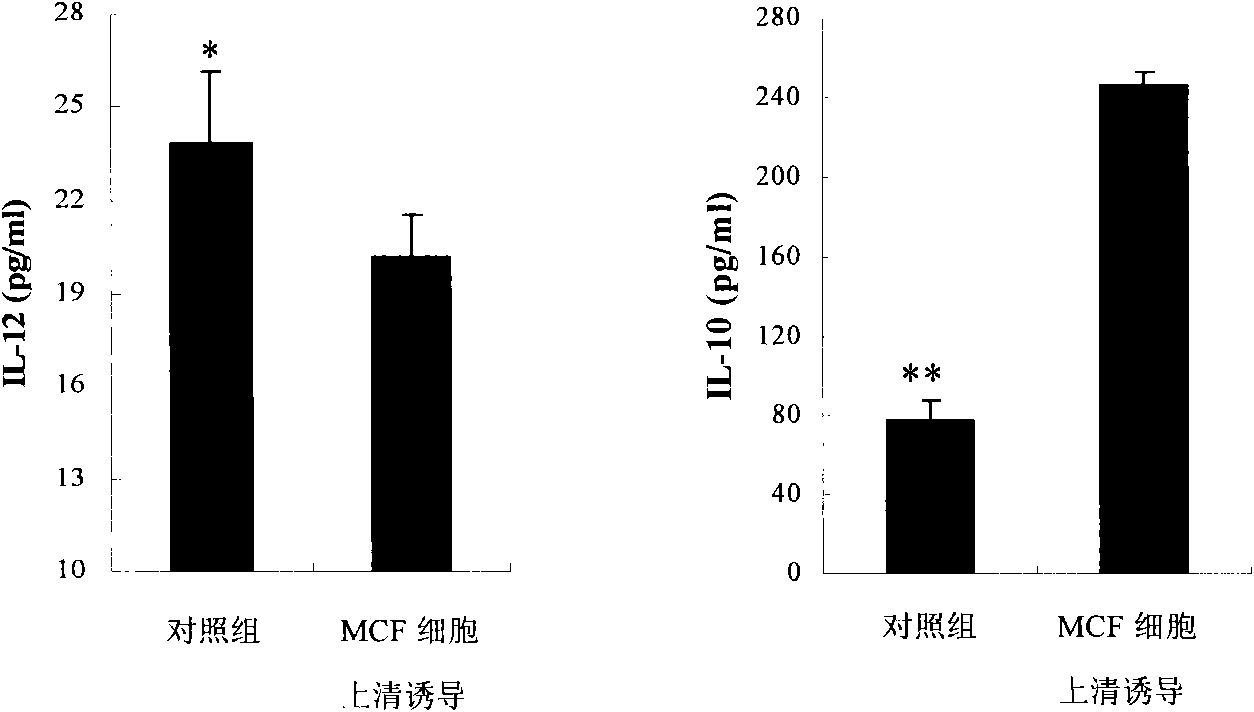
[0058] Example 2. Immunomodulatory effect of heterologous protein neu-Fc combined with IFN-γ and BCG
[0059] 1. The combined effect of neu-Fc, IFN-γ and BCG on the secretion of cytokines
[0060] Adjust the density of PBMC to 2×10 with phenol red-free RPMI-1640 medium containing 20% serum 6 / ml, added to 96-well cell culture plate, 100μl per well. In the experimental group, 100μl of MCF-7 cell culture supernatant was added. After 48-72 hours of culture, the culture solution was aspirated and divided into the following treatments: 1. Supplement RPMI-1640100μl; 2. Add neu-Fc containing a final concentration of 10nM 100μl of phenol red-free RPMI-1640 medium; 3. Add 100μl of phenol red-free RPMI-1640 medium with a final concentration of 40U / ml IFN-γ; 4. Add a final concentration of 40U / mlIFN-γ and 10nM neu -Fc-free phenol red RPMI-1640 medium 100μl; 5. Add 100μl of phenol red-free RPMI-1640 medium containing a final concentration of 300μg / ml BCG; 6. Add a final concentration of 300...
Embodiment 3
[0085] Example 3. Animal experiment
[0086] 1. Construction of EMT6 / Her2 cells
[0087] The mouse breast cancer cells EMT6 (purchased from ADCC, USA) derived from Balb / C mice were transfected with pcDNA3 / Her2 plasmid, and EMT6 cells expressing Her2 were selected under the pressure of 800μg / ml G418. After two limiting dilutions and pressure After screening, a clone of EMT6 cells stably expressing Her2 was obtained and named EMT6 / Her2. At the same time, EMT6 cells not transfected with pcDNA3 / Her2 plasmid were used as control. The anti-Her2 antibody (Ab20) was purchased from Neomarkers and FACS was used to detect the expression of Her2 in EMT6 / Her2 cells. The FACS test results are as follows Picture 8 Shown. among them, Picture 8 A is the FACS test result of EMT6 cells in the control group, Picture 8 B is the FACS test result of EMT6 / Her2 cells. The results showed that nearly 100% of EMT6 / Her2 cells express Her2 molecules.
[0088] 2. Animal experiment
[0089] 1) Determination o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com