Synthesis process of yellow dye of leather
A synthetic process, the technology of yellow dyes, applied in the field of synthetic process of dyes, can solve the problems of large migration, poor color fastness, etc., and achieve the effect of light chroma, high dyeing rate and saving money
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
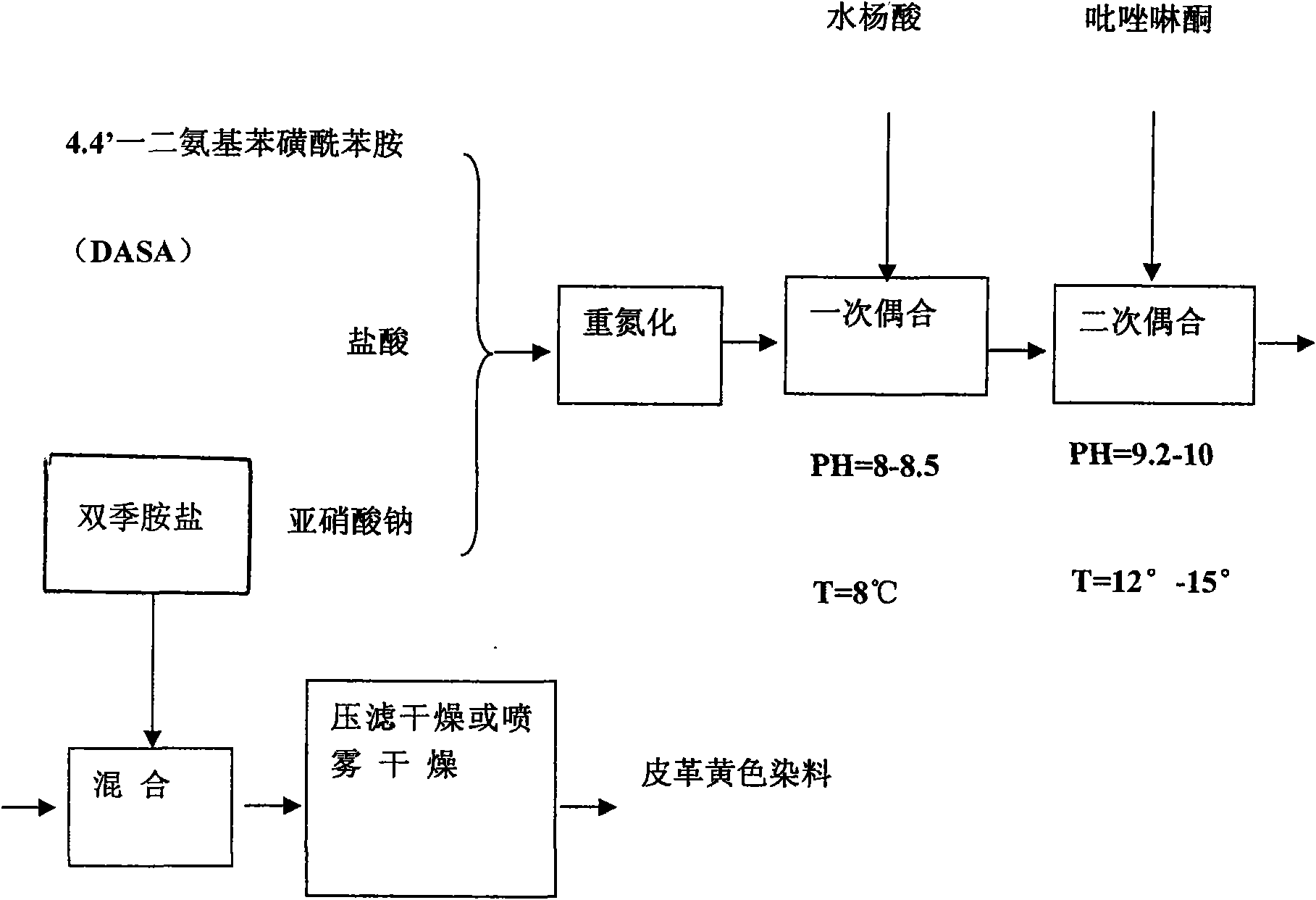
[0019] According to the following molar ratio: 4.4'-diaminobenzenesulfonanilide: HCl: NaNO 2 : salicylic acid: pyrazolone = 1: 5.6: 2.2: 1: 0.98, mix 4.4'-diaminobenzenesulfonanilide, hydrochloric acid, and sodium nitrite at a temperature T≤12°C, 0.5≤PH≤ Under the conditions of 2.5, the reaction time is 0.5-2.5 hours for diazotization; then add salicylic acid, and under the conditions of temperature 3-16 ° C, 6≤PH≤10, the reaction time is 2-4.5 hours for a coupling; add Pyrazolone, under the conditions of temperature 8-22°C, 8≤PH≤11, reaction time 2-5 hours for secondary coupling; after secondary coupling, add bis-quaternary ammonium salt and mix; mix and dry to produce finished product .
Embodiment 2
[0021] According to the following molar ratio: 4.4'-diaminobenzenesulfonanilide: HCl: NaNO 2 : salicylic acid: pyrazolone = 1: 5.6: 2.2: 1: 0.98, mix 4.4'-diaminobenzenesulfonanilide, hydrochloric acid, and sodium nitrite at a temperature of 5-10°C, 0.8≤ Under the condition of PH≤2.2, the reaction time is 1-2 hours for diazotization; then add salicylic acid, and under the condition of temperature 5-15℃, 8.5≤PH≤9.5, the reaction time is 2.5-4 hours for a coupling ; add pyrazolone, under the conditions of temperature 10-20 ℃, 8.5≤PH≤10, reaction time 3-4.5 hours for secondary coupling; after secondary coupling, add double quaternary ammonium salt and mix; mix and dry to produce Finished product.
Embodiment 3
[0023] According to the following molar ratio: 4.4'-diaminobenzenesulfonanilide: HCl: NaNO 2 : salicylic acid: pyrazolone = 1: 5.6: 2.2: 1: 0.98, mix 4.4'-diaminobenzenesulfonanilide, hydrochloric acid, and sodium nitrite at a temperature of 8-12°C, 1≤ Under the condition of PH≤2.5, the reaction time is 1-1.5 hours for diazotization; then add salicylic acid, and under the condition of temperature 8-12℃, 8.2≤PH≤9.8, the reaction time is 2-4.5 hours for a coupling ; add pyrazolone, under the condition of temperature 15-20 ℃, 8.2≤PH≤9.6, the reaction time is 3.5-4.5 hours for secondary coupling; after secondary coupling, add double quaternary ammonium salt and mix; mix and dry to produce Finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com