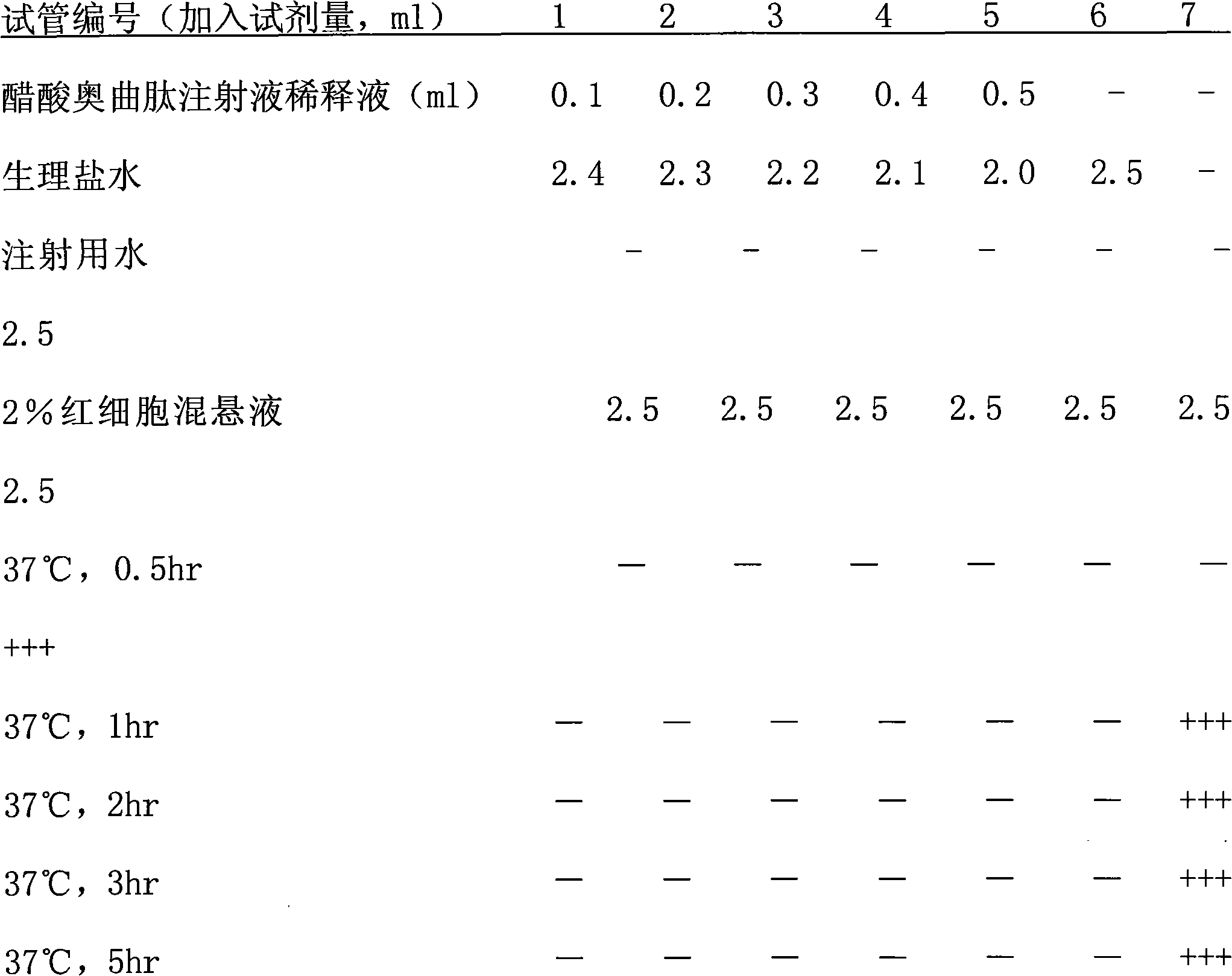
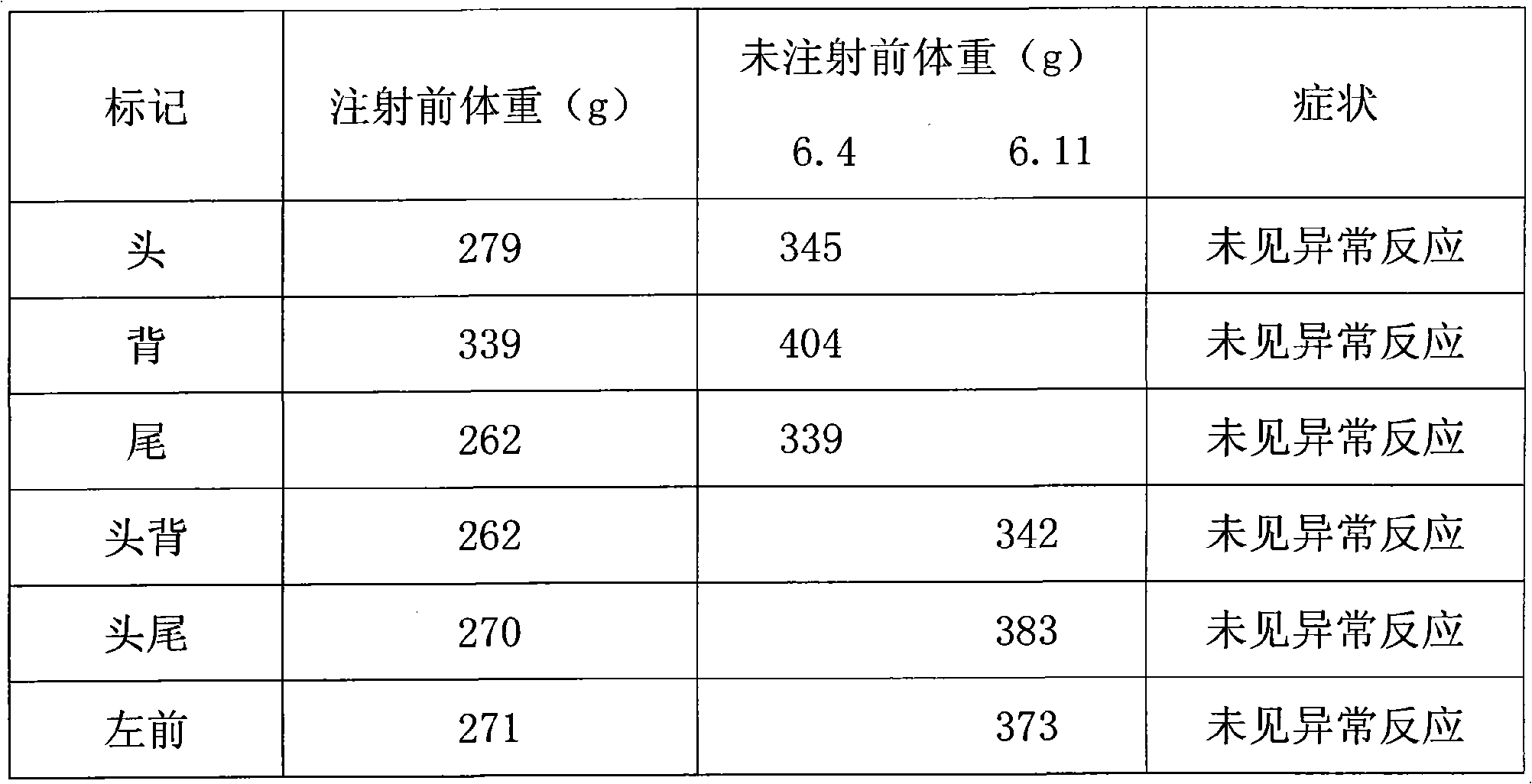
Octreotide acetate injection
A technology of octreotide acetate and injection, applied in the field of medicine, can solve the problems of instability, unfavorable storage and transportation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Preparation of Octreotide Acetate Injection
[0014] Prescription (calculated in 1000 sticks):
[0015] Octreotide Acetate 1.2g
[0016] Mannitol 8 grams
[0017] Tween - 800.001 grams
[0018] Citric acid 0.1 g
[0019] Add water for injection to 1000ml
[0020] Preparation process: add 8 grams of mannitol and dissolve in 100 ml of water for injection, then add 0.001 grams of Tween-800, add 1.2 grams of octreotide acetate after dissolving, measure the pH, add 0.1 grams of citric acid to adjust the pH to between 4.0 and 4.5, and then Add water for injection to 1000 ml.
[0021] After the medicinal solution is prepared, it is aseptically filtered through a 0.22 micron sterilizing film, and then filled according to 1 ml of the preparation, plugged after filling, capped, and packaged after light inspection.
Embodiment 2
[0023] Prescription (calculated in 1000 sticks):
[0024] Octreotide Acetate 1.2g
[0025] Mannitol 12 grams
[0026] Tween - 800.0015 grams
[0027] 0.5 g citric acid
[0028] Add water for injection to 1000ml
[0029] Preparation process is with embodiment 1
Embodiment 3
[0031] Prescription (calculated in 1000 sticks):
[0032] Octreotide Acetate 1.2g
[0033] Mannitol 10g
[0034] Tween - 800.0012 grams
[0035] 0.3 g citric acid
[0036] Add water for injection to 1000ml
[0037] Preparation process is with embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com