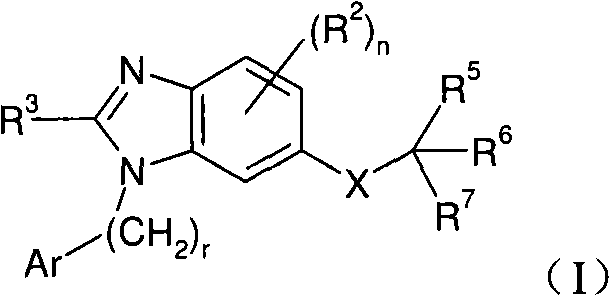
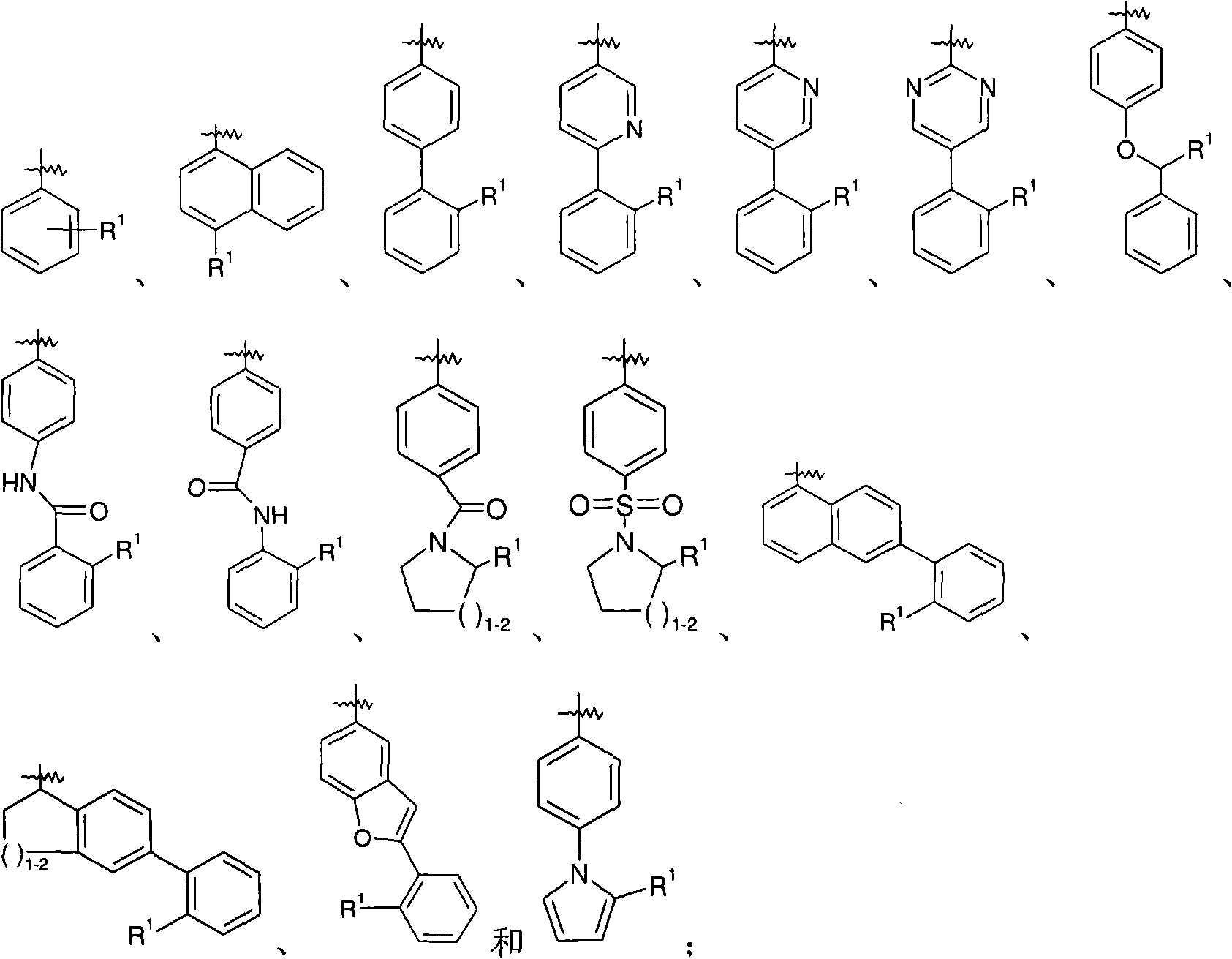
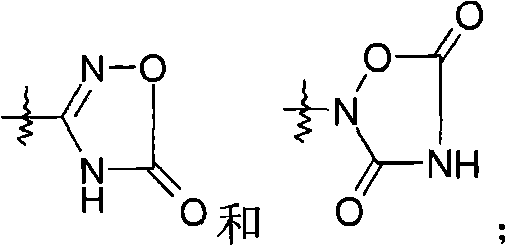
Dual-acting benzoimidazole antihypertensive agents
A technology of alkyl and alkylene, applied in the field of novel compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0365] Preparation of Aryl Compounds
[0366]
[0367] Compound (1d) can be prepared using synthetic methods reported in literature such as Duncia et al., (1991) J.Org.Chem. 56:2395-400 and among others References cited. Alternatively, the starting material (1e) is commercially available in protected form. Using commercially available unprotected starting material (1d), first protect R 1 group to form a protected intermediate (1e), followed by addition of a leaving group (L), such as by halogenation, to form compound (1f). For example, bromination of the methyl group of N-triphenylmethyl-5-[4'-methylbiphenyl-2-yl]tetrazole is described in Cao et al., (2005) Chinese Chemical Huihuizhi (J.Chinese Chem.Soc.) 52:539-544. In addition, when Ar * Having a -CN group can then be converted to the desired tetrazolyl, which can be protected. Nitrile groups are readily converted by reaction with suitable azides such as sodium azide, trialkyltin azide (especially tributyltin azide)...
example
[0514] The following Preparations and Examples are provided to illustrate specific embodiments of the invention. However, these specific examples are not intended to limit the scope of the invention in any way unless specifically indicated otherwise.
[0515] Unless otherwise indicated, the following abbreviations have the following meanings, and any other undefined abbreviations used herein have their standard meanings:
[0516] ACE angiotensin converting enzyme
[0517] APP aminopeptidase P
[0518] AT 1 Angiotensin II type 1 (receptor)
[0519] AT 2 Angiotensin II type 2 (receptor)
[0520] BSA bovine serum albumin
[0521] DCM dichloromethane
[0522] DIPEA N,N-Diisopropylethylamine
[0523] DMF N,N-Dimethylformamide
[0524] DMSO Dimethyl Sulfoxide
[0525] Dnp 2,4-Dinitrophenyl
[0526] DOCA deoxycorticosterone acetate
[0527] EDC N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride
[0528] EDTA ethylenediaminetetraacetic acid
...
example 1
[0566] 4'-[6-((R)-1-Benzyl-2-hydroxycarbamoylethylcarbamoyl)-4-methyl-2-propylbenzimidazol-1-yl Methyl]biphenyl-2-carboxylic acid tert-butyl ester (1a; R 1a = tert-butyl) and 4'-[6-((R)-1-benzyl-2-hydroxycarbamoyl ethylamine Formyl)-4-methyl-2-propylbenzimidazol-1-ylmethyl]biphenyl-2-carboxylic acid (1b; R 1a =H)
[0567]
[0568] Dichloromethane (20 mL) and TFA (30 mL) were added to (R)-tert-butyl 1-benzyl-2-hydroxycarbamoylethyl)carbamate (2.8 g, 9.5 mmol; prepared as described in Preparation 4 prepared) solution. The mixture was stirred at room temperature for 30 minutes and concentrated to dryness under reduced pressure. The solid was dissolved in DMF (60 mL), followed by the addition of 3-(2'-tert-butoxycarbonylbiphenyl-4-ylmethyl)-7-methyl-2-propyl-3H-benzimidazole-5- Formic acid (2.6 g, 5.4 mmol; prepared as described in Preparation 3), HOBt (1.3 g, 10 mmol), EDC (1.8 g, 10 mmol) and DIPEA (3.9 mL, 22.4 mmol). The final mixture was stirred overnight ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com