Novel use of dipeptidyl peptidase-iv inhibitor
An enzyme activity inhibitor, dipeptidyl peptidase technology, applied in the direction of organic active ingredients, non-central analgesics, anti-inflammatory agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
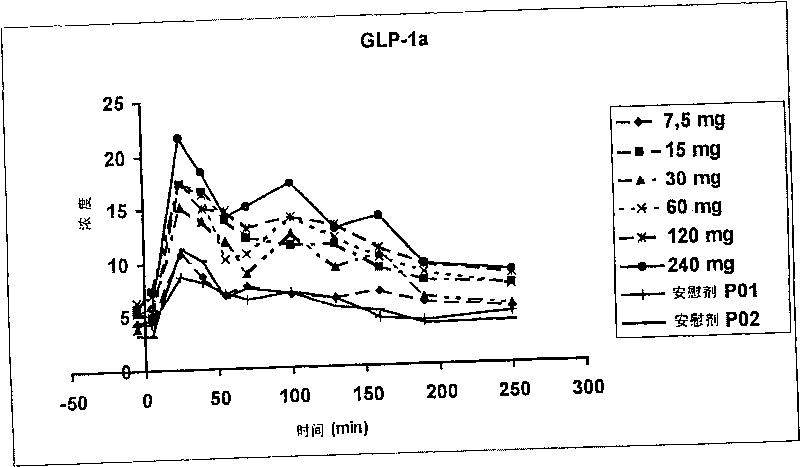
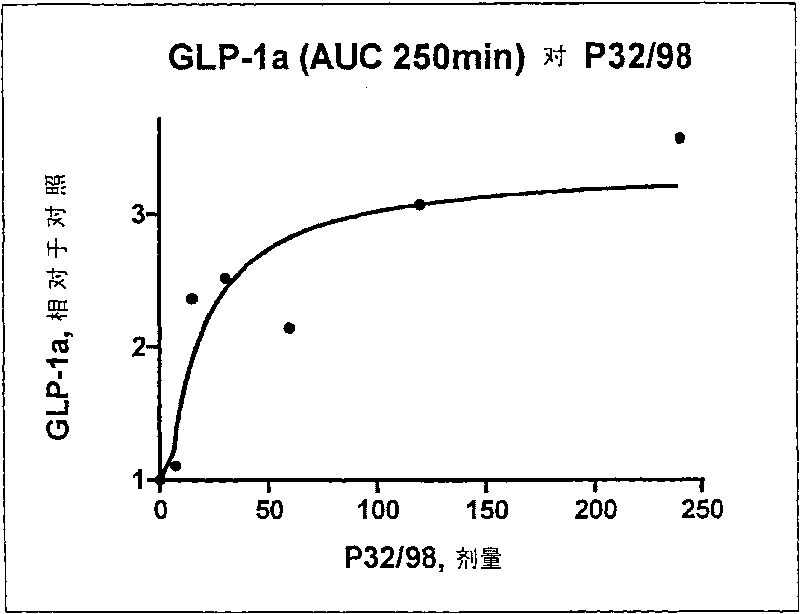
[0038] The DP IV inhibitor P32 / 98 is transported via the activity of the PepT1 intestinal peptide transporter. The rapid and active transport of P32 / 98 across the intestinal mucosa is responsible for its fast onset. t max is a prerequisite for an efficient target of dipeptidyl peptidase IV (DP IV). Oral administration of P32 / 98 resulted in maximal target inhibition at 15 to 20 minutes and 30 to 40 minutes after ingestion in rats and humans, respectively. Therefore, DP IV inhibitors should be given 10-20 minutes before glucose or food intake.
[0039] In a first-in-human study with P32 / 98, pharmacokinetic parameters, such as insulin and GLP-1 concentrations in plasma, and blood glucose were studied in 36 healthy male volunteers. Oral doses of P32 / 98 are in the following concentrations: 7.5 mg, 15 mg, 30 mg, 60 mg, 120 mg and 240 mg. The results for the above pharmacokinetic parameters are summarized in Table 1.
[0040] 36 healthy male subjects were divided into 3 individu...
Embodiment 2
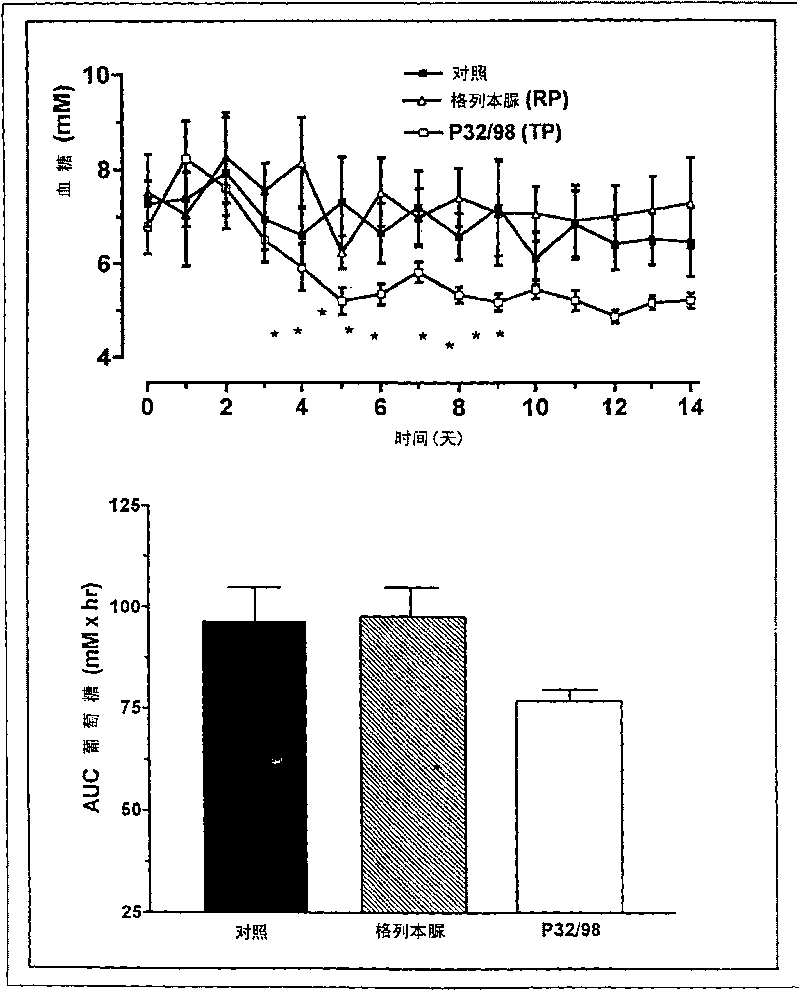
[0063] P32 / 98 nutrient dependence supports initial insulin secretion in obese Zucker rats. However, P32 / 98 decreased total daily insulin secretion during subchronic treatment. P32 / 98 caused insulin savings of 45% compared to the control glibenclamide which increased insulin output by 27%.
[0064] Trials were performed to determine whether P32 / 98 is the first choice to affect glucose tolerance in vivo by increasing the circulating half-life of the incretins GIP and GLP-1. Glibenclamide (Maninil Berlin-Chemie, Berlin, Germany) as a reference material for comparative studies. Glibenclamide is one of the most effective drugs for lowering blood sugar in patients with type II diabetes and is one of the most commonly prescribed sulfonylureas.
[0065] Male Zucker fa / fa rats, which exhibit abnormalities in glucose metabolism and are an ideal animal model for type II diabetes, were studied in the following manner:
[0066] P32 / 98 and glibenclamide were given once daily for 21 day...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com