Marine low temperature alpha-amylase gene engineering bacteria, recombinant enzyme and application
A technology of genetically engineered strains and amylase, which is applied in the field of bioengineering, can solve the problems of reduced efficiency of microbial decomposition of organic pollutants, etc., and achieves the effects of low acting temperature, simple preparation process and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
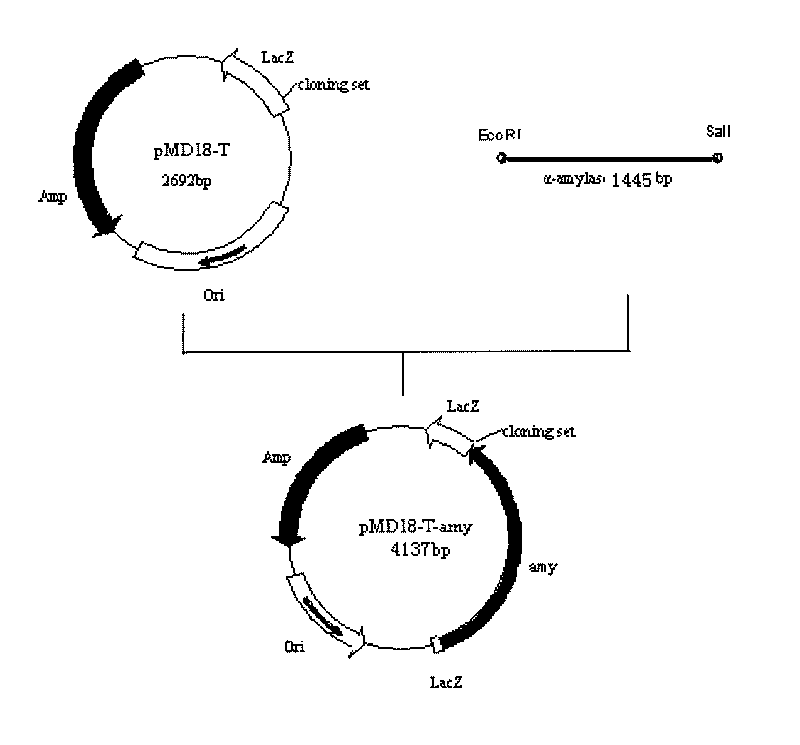
[0039] Example 1. Construction of the cloning plasmid pMD18-T-amy.
[0040] Low-temperature α-amylase-based primers, forward primer 1 and reverse primer 2, were designed. An EcoR I restriction site was added to primer 1, and a Sal I restriction site was added to primer 2.
[0041] Primer 1 sequence: 5'-GCGC GAATTC ATGAACAGGGGTATAT-3', the underlined part is the EcoR I site.
[0042] Primer 2 sequence: 5'-TA GTC GAC The underlined part of TCAGCCCACCCCACAG-3' is the Sal I site.
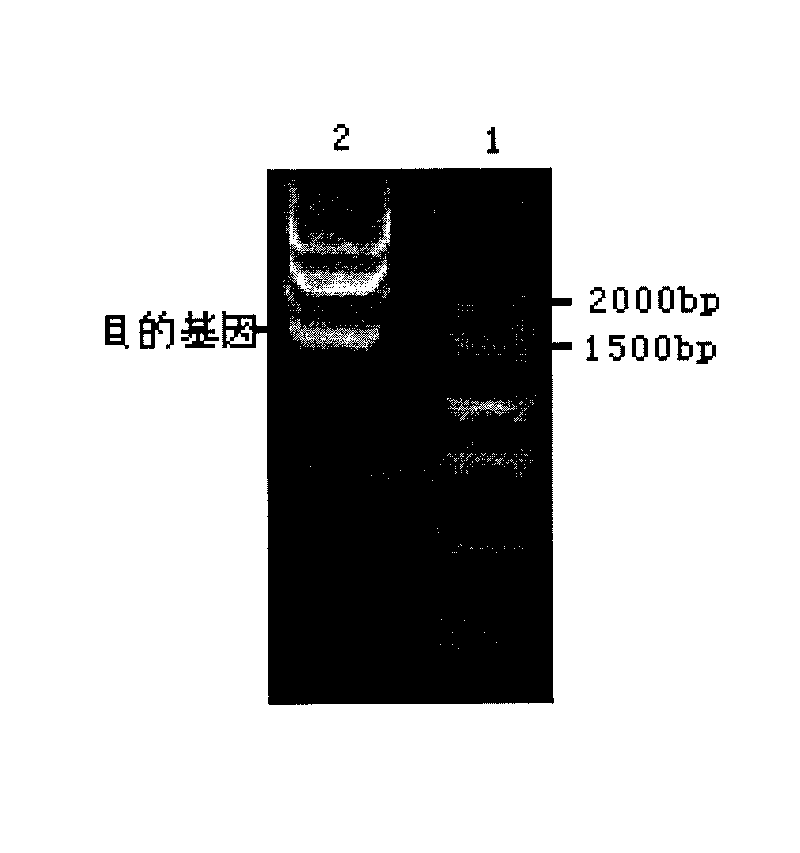
[0043] The genomic DNA of Pseudoalteromonas arctica GS230 was used as a template for PCR amplification, and the PCR product was electrophoresed, recovered and purified by tapping the gel with the Gel Extraction Kit kit from TaKaRa Company. Linked with the pMD18-T vector, the cloning plasmid pMD18-T-amy was constructed (see figure 1 ), transform Escherichia coli competent cells, and initially screen positive clones through blue-white colonies, and then carry out electrophoresis detection after do...
Embodiment 2
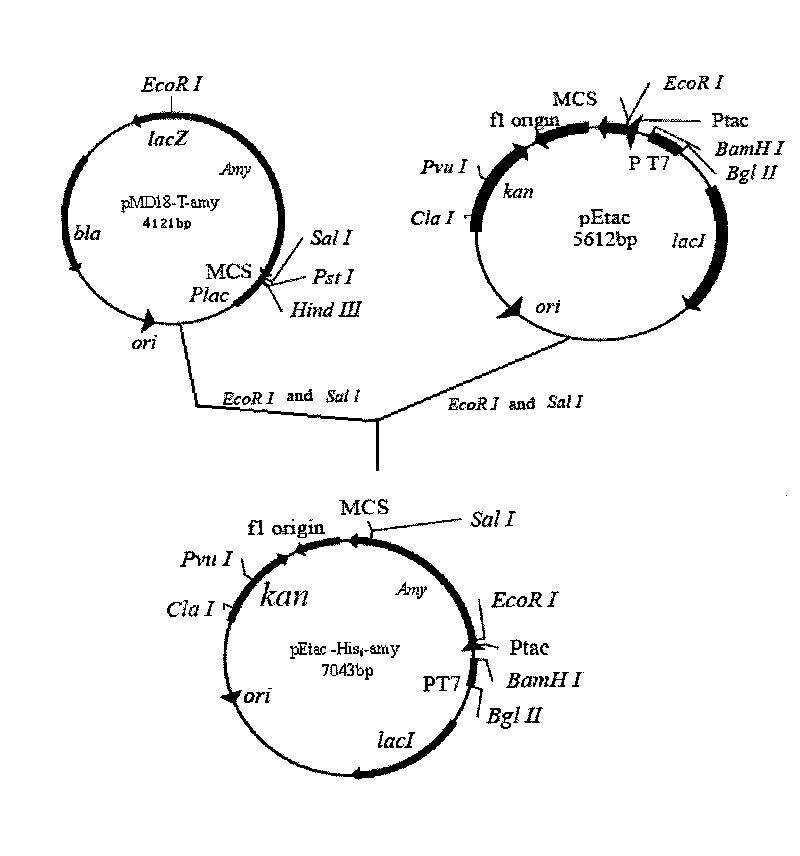
[0044] Example 2. Expression vector pEtac-His 6 -amy's build.
[0045] Recombinant T-vector (pMD18-T-amy) and pEt-28a-His 6 Carry out EcoR I and Sal I double enzyme digestion respectively, and the enzyme digestion reaction system (20 μ L) is as follows:
[0046] 10×buffer H 2μL
[0047] EcoR I (or Sal I) (10U·μL -1 ) 1μL
[0048] pEtac-His 6 (or PCR product) 14μL
[0049] Carry out EcoR I and Sal I double digestion of pMD18-T-amy, and recover a 1.5kb band from the gel; perform EcoR I and Sal I double digestion on the pEac plasmid, and recover a 5.6kb band from the gel; The products were mixed, and connected at 16°C for 18h (such as image 3 ), the expression vector pEtac-His containing six histidine tags at the N-terminal was constructed 6 -amy. When exogenous genes are expressed in E.coli, in order to simplify the purification steps of the expression products, tags such as His and MBP are usually added so that the expression products can be purified by Ni-NTA chroma...
Embodiment 3
[0050] Example 3. A kind of genetically engineered strain Escherichia coli EPGS230 (Escherichia coli EPGS230) CGMCC No.2700 for expressing the recombinant low-temperature α-amylase recombinant expression vector described in Example 2, it is the expression described in Example 2 Vector pEtac-His 6 - The genetically engineered strain Escherichia coli EPGS230 (Escherichia coli EPGS230) CGMCC No.2700 obtained by transforming amy into Escherichia coli BL21 (DE3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com