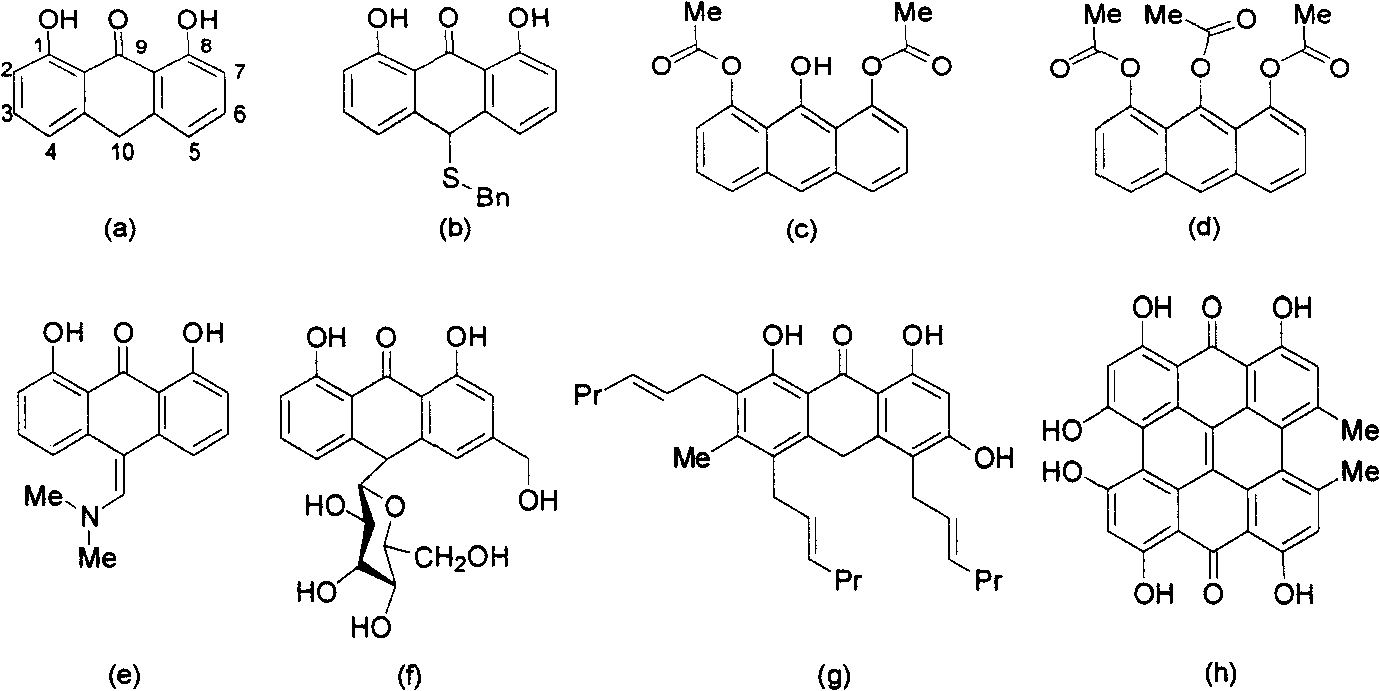
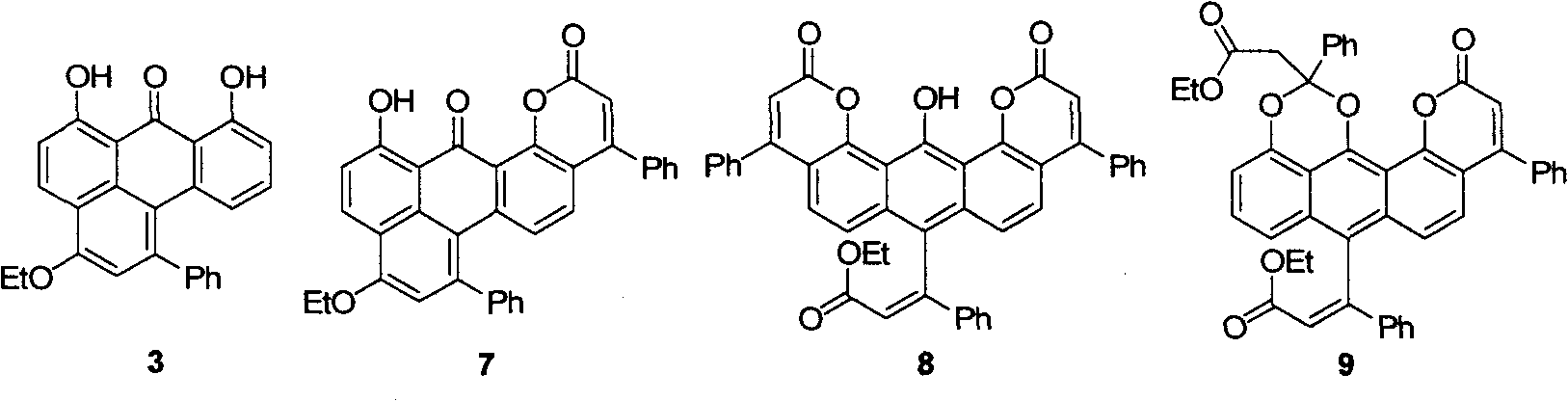
Anthralini-fused ring derivatives and synthesis method thereof
A synthesis method and derivative technology, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., to achieve the effects of simple operation, mild reaction conditions, and excellent yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
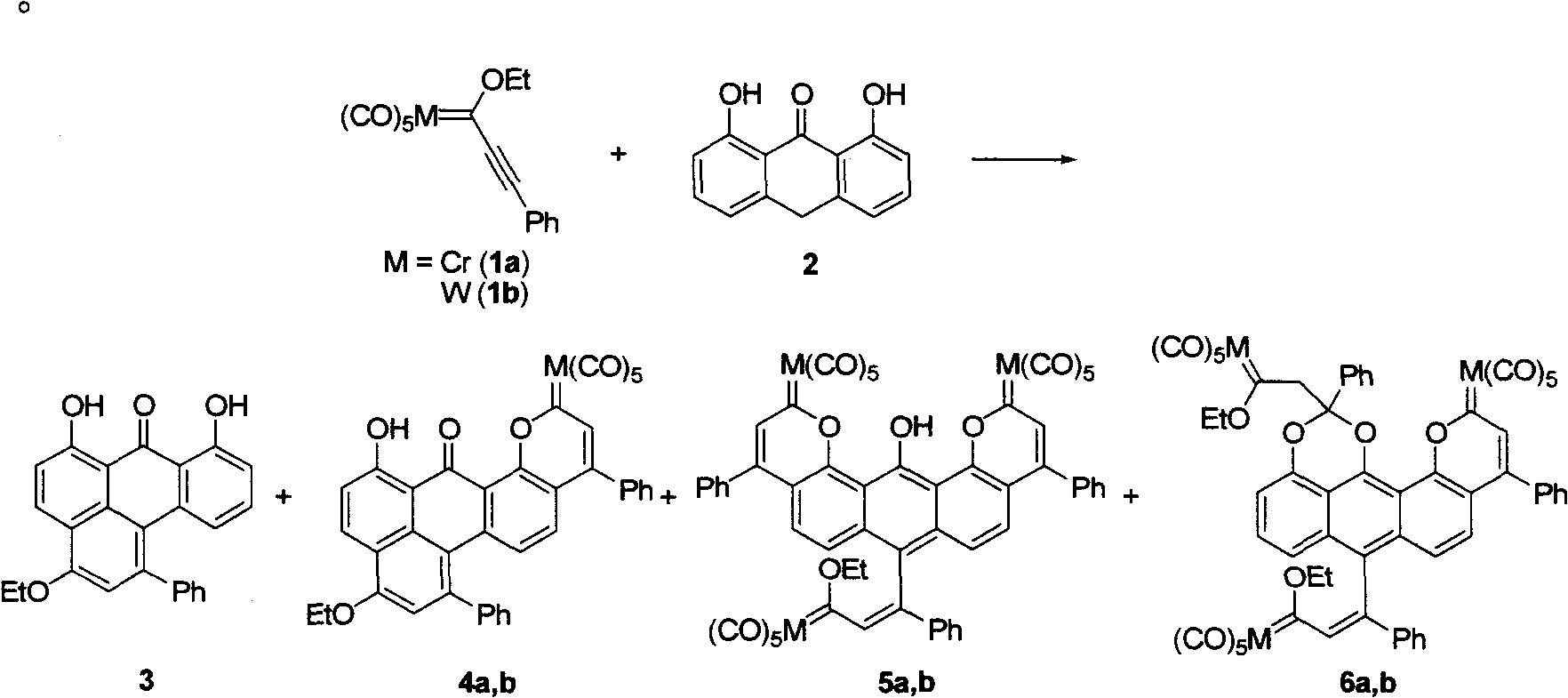
[0048] Example 1: Fischer chromium carbene and anthralin react in toluene at a ratio of 1:1
[0049]In a 25mL Schlenk reaction flask, add 1-phenylethynyl Fischer chromium carbene compound (1a) (175mg, 0.5mmol), anthralin (2) (114mg, 0.5mmol), N 2 Replace three times, inject 5 mL of toluene and 104 μL (0.75 mmol) of triethylamine, and stir at room temperature for 1 h. Separation and purification by column chromatography, the chromatographic column is a silica gel column, and the eluent is petroleum ether (boiling range 30-60°C) / dichloromethane (v / v, 2:1, 1:1 gradient change), according to the flow out sequence Sequentially collect orange-red and green components successively, obtain compound (3) respectively after removing solvent [(48mg, yield is 25%, R f =0.5, petroleum ether (boiling range 30-60°C) / dichloromethane (v / v, 2:1); high-resolution mass spectrometry confirmed the product, x-ray crystallography confirmed the absolute configuration of the product, and NMR verified t...
Embodiment 2
[0050] Example 2: Fischer tungsten carbene and anthralin react in toluene at a ratio of 1:1
[0051] In a 25mL Schlenk reaction flask, add 1-phenylethynyl Fischer chromium carbene compound (1b) (241mg, 0.5mmol), anthralin (2) (114mg, 0.5mmol), N 2 Replaced three times, injected 5 mL of toluene and 104 μL (0.75 mmol) of triethylamine, and stirred at room temperature for 3.5 h. Separation and purification by column chromatography, the chromatographic column is a silica gel column, the eluent is petroleum ether (boiling range 30-60°C) / dichloromethane (v / v, 1:1), the orange-red components are collected, and after removing the solvent Obtain product (3) [(88mg, yield is 46%, R f =0.4, sherwood oil (boiling range 30~60 ℃) / dichloromethane (v / v, 2:1); NMR verification compound purity>95% (same as Example 1), its high-resolution mass spectrum and NMR data are listed in After the examples].
Embodiment 3
[0052] Example 3: Fischer chromium carbene reacts with anthralin at a ratio of 5:1 in dichloromethane
[0053] In a 25mL Schlenk reaction flask, add 1-phenylethynyl Fischer chromium carbene compound (1a) (578mg, 1.65mmol), anthralin (2) (74mg, 0.33mmol), N 2 Replaced three times, injected 5 mL of dichloromethane and 75 μL (0.5 mmol) of triethylamine, and stirred at room temperature for 19 h. Separation and purification by column chromatography, the chromatography column is a silica gel column, and the eluent is petroleum ether (boiling range 30-60°C) / dichloromethane (v / v, 5:1, 3:1, 1:1 gradient change) , collect brown-green, brown and green three components successively according to the order of flowing out, obtain product (4a) respectively after removing solvent [(134mg, yield is 33%, R f =0.6, petroleum ether (boiling range 30~60°C) / dichloromethane (v / v, 2:1); NMR and elemental analysis verify compound purity>95%], (5a)[(98mg, yield is 25% %,R f =0.5, petroleum ether (boi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com