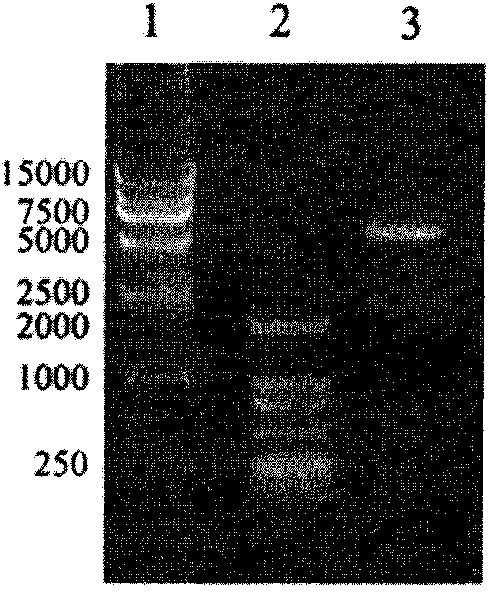DNA vaccine for promoting regeneration and functional rehabilitation of nerves of central system
A DNA vaccine and nerve regeneration technology, applied in the field of medicine, can solve problems such as unsatisfactory, unsuitable for human beings, and unable to block inhibitory molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] In order to better understand the present invention, the preparation process of the DNA vaccine of the present invention and its technical effects will be described in detail below through specific examples.
[0029] 1. Reagents required for DNA vaccine construction and identification of the present invention
[0030]The LINGO-1 gene was purchased from Openbiosystem Company (Catalog Number: MHS1010-73556, and the vector was pCMV-SPORT6); the TN-R and neurocan domains were synthesized from the whole gene of Shanghai Invitrogen Company and connected to the pMD-18T vector, and the designed specificity The primers are also synthesized by the company (because the fragments are not long, in order to save time and prevent unnecessary base mutations when routinely catching genes, we use the whole gene synthesis method);
[0031] PCR Amplification Kit, DNA Marker (DL 2000, DL15000), Protein Marker and DAB Chromogenic Substrate were purchased from TaKaRa Company; PCR Purification...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com