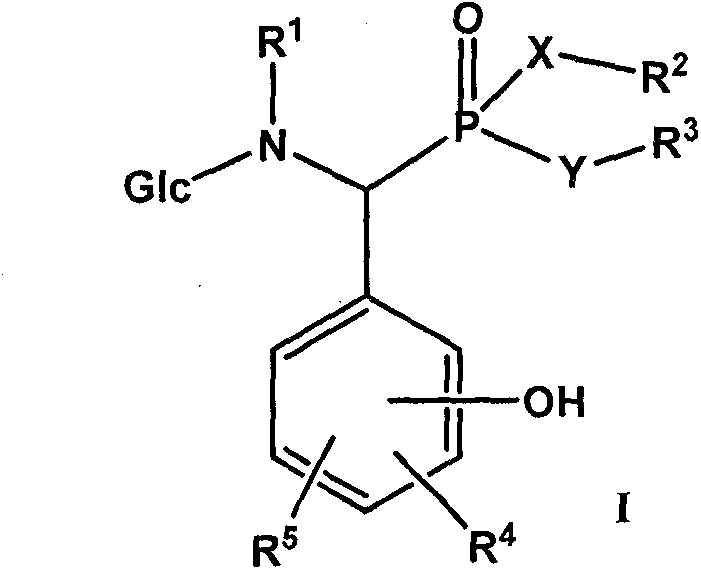
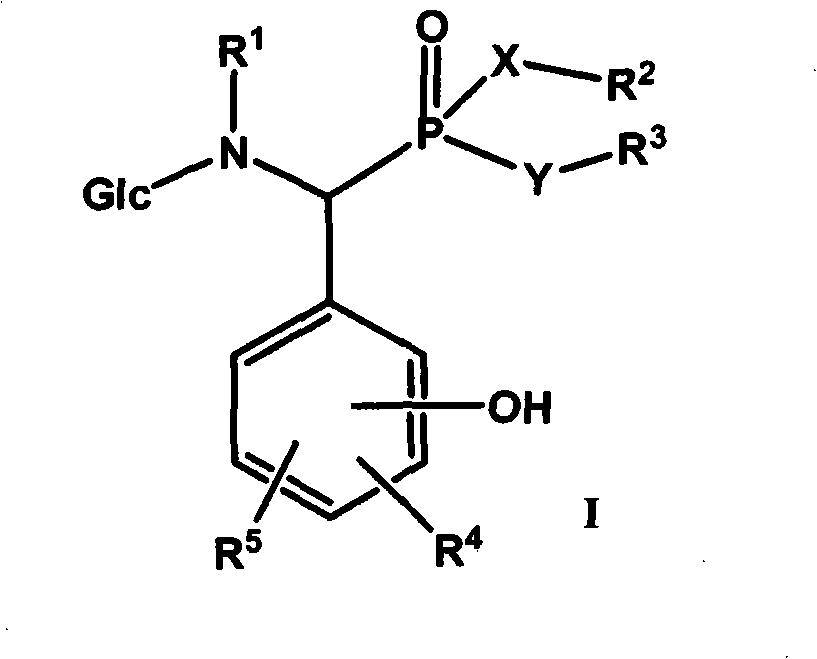
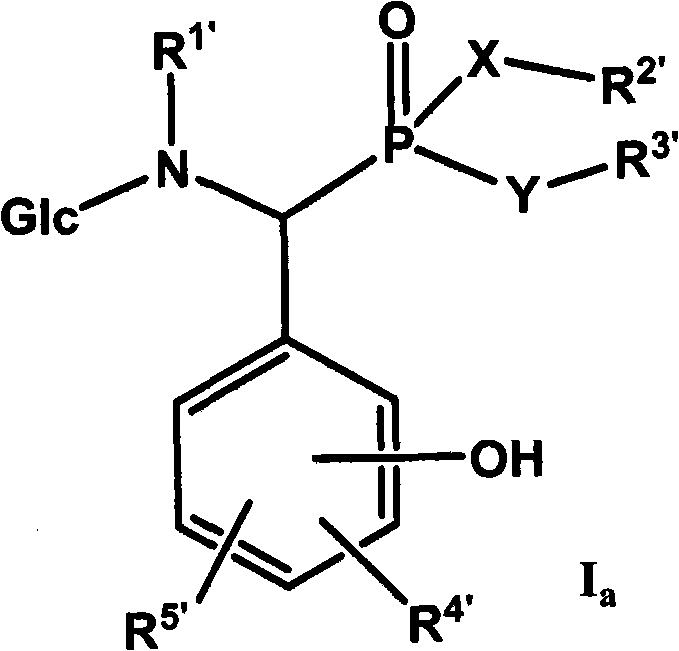
Derivative of alpha-amino phosphonic acid containing saccharide groups, preparation method and medical application thereof
A technology of methyl amino group and methyl methyl phosphonic acid monoethyl ester, which is applied in the field of glycosyl-containing α-amino phosphonic acid derivatives, its preparation and its medical application, and can solve the lack of prevention and/or prevention of autoimmune diseases or treatment, immunocompromised, Cushing's syndrome, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1: Preparation of α-[N-(D-glucosyl)-N-methylamino]-α-(2,4-dihydroxyphenyl)methylphosphonic acid monoethyl ester-compound 1
[0090] Weigh 7.00g (0.0507mol) 2,4-dihydroxybenzaldehyde, 9.80g (0.0502mol) N-methyl-D-glucosamine and 7.80g (0.0565mol) diethyl phosphite, add 60ml absolute ethanol Dissolve, stir and react at a bath temperature of about 65°C for 5 days, cool, concentrate under reduced pressure, add ethyl acetate, a yellow solid precipitates, collect the solid by filtration, wash with ethyl acetate, dry to obtain 20.20g, yield 94.6%, mp 190 -193°C (dec.).
[0091] 1 H-NMR (D 2 O, ppm) δ: 0.969(t, J=7.00Hz, 3H), 2.136(d, J=8.82Hz, 2H), 2.538(s, 3H), 2.90-3.10(m, 2H), 3.40-4.00( m, 11H), 4.42-4.48 (br-s, 1H), 6.204 (d, J=2.24Hz, 1H), 6.267 (dd, J 1 =8.40Hz,J 2 = 2.24Hz, 1H), 6.956 (d, J = 8.40Hz, 1H).
Embodiment 2
[0092] Example 2: Preparation of α-[N-(D-glucosyl)-N-methylamino]-α-(2,3-dihydroxyphenyl)methylphosphonic acid monoethyl ester-compound 2
[0093] According to the method of Example 1, 2,3-dihydroxybenzaldehyde was used instead of 2,4-dihydroxybenzaldehyde to prepare it, with a yield of 76.0%, mp 170-173°C (dec.).
[0094] 1 H-NMR (D 2 O, ppm) δ: 9.49(t, J=7.00Hz, 3H), 2.170(d, J=7.92Hz, 2H), 2.525(s, 3H), 2.90-3.10(m, 2H), 3.38-3.96( m, 11H), 4.40-4.47 (br-s, 1H), 6.58-6.78 (m, 3H).
Embodiment 3
[0095] Example 3: Preparation of α-[N-(D-glucosyl)-N-methylamino]-α-(3,4-dihydroxyphenyl)methylphosphonic acid diethyl ester-compound 3
[0096] According to the method of Example 1, 3,4-dihydroxybenzaldehyde is used instead of 2,4-dihydroxybenzaldehyde to prepare the reaction, the yield is 85.4%, mp 196-199°C (dec.).
[0097] 1 H-NMR (D 2 O, ppm) δ: 0.85-1.16 (m, 6H), 2.42-2.67 (m, 5H), 2.82-3.12 (m, 2H), 3.40-4.00 (m, 14H), 6.50-6.90 (m, 3H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com