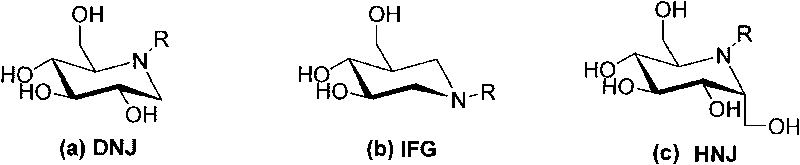
Azasugars compounds, synthesis method and applications thereof
A technology for compounds and carbohydrates, applied in the fields of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve the problems of low overall yield, cumbersome preparation process, unclear structure-activity relationship research, etc., to achieve strong cellular immunity and Humoral immune activity, the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
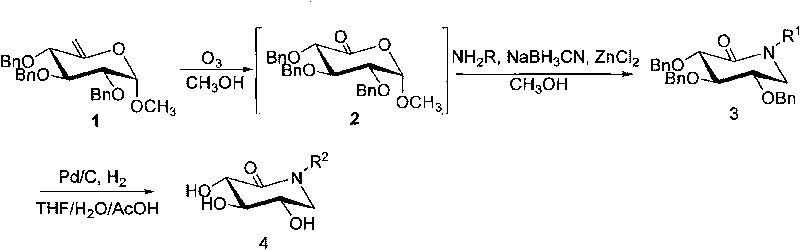
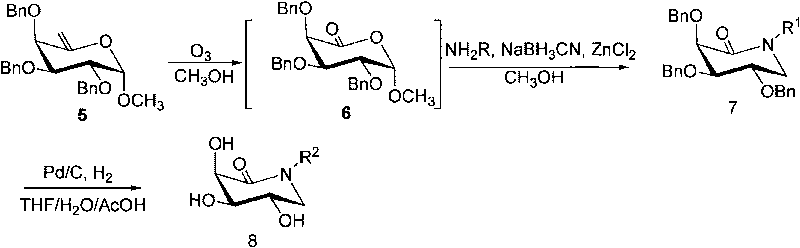
[0044] Synthesis of Example 1 Compound 3a ((3S, 4R, 5S)-3,4,5-three (benzyloxy)-1-n-decylpiperidin-2-one)
[0045] Compound 1 ((2S, 3R, 4S, 5S)-3,4,5-tris(benzyloxy)-tetrahydro-2-methoxy-6-vinyl-dihydropyran) (200 mg, 0.45mmol) was dissolved in anhydrous methanol, cooled to -78°C, and slowly passed into O 3 Gas until the reaction solution turns blue. After stirring at -78°C for 3 minutes, N was slowly introduced into the 2 Until the blue color disappears, add n-decylamine (178 μl, 0.90 mmol), NaCNBH 3 (59mg, 0.9mmol) and ZnCl 2 (12mg, 0.09mmol), reflux reaction 2h, add saturated NaHCO under ice-cooling 3 Quenches the reaction. Methanol was distilled off under reduced pressure, the residue was dissolved in ethyl acetate (80m), washed twice with saturated NaCl solution, anhydrous NaCl 2 SO 4 After drying, filter. The filtrate was concentrated under reduced pressure to remove ethyl acetate, and the residue was separated and purified by normal pressure column chromatograph...
Embodiment 2
[0048] Synthesis of Example 2 Compound 3b ((3S, 4R, 5S)-3,4,5-three (benzyloxy)-1-n-nonylpiperidin-2-one)
[0049] It is prepared from compound 1 and n-nonylamine, and the specific operation steps are the same as the synthesis of compound 3a. Atmospheric column separation [eluent (V / V) petroleum ether: ethyl acetate = 8:1]. Product properties: white solid, yield 90%. 1 H NMR (300MHz, CDCl 3 )δ0.88(t, 3H, J=6.9Hz), 1.24(s, 12H), 1.49(s, 2H), 3.22-3.44(m, 4H), 3.72(dd, 1H, J=6.3, 11.1Hz ), 3.82(t, 1H, J=6.9Hz), 3.98(d, 1H, J=6.9Hz), 4.55-4.19(m, 5H), 5.12(d, 1H, J=11.1Hz), 7.26-7.44 (m, 15H); 13 C NMR (75MHz, CDCl 3 )δ14.1, 22.6, 26.8, 27.3, 29.2, 29.3, 29.4, 31.8, 47.0, 47.8, 72.1, 73.7, 74.1, 76.2, 79.4, 82.1, 127.6, 127.7, 127.8, 127.9, 128.2, 128.3, 128.7, 1 , 137.9, 138.0, 168.4; HRMS: Calcd for C 27 h 28 o 6 [M+Na] + , 566.3241; Found, 566.3240.
Embodiment 3
[0050] Synthesis of Example 3 Compound 3c ((3S, 4R, 5S)-3,4,5-three (benzyloxy)-1-n-octylpiperidin-2-one)
[0051] Prepared from compound 1 and n-octylamine, the specific operation steps are the same as the synthesis of compound 3a. Atmospheric column separation [eluent (V / V) petroleum ether: ethyl acetate = 8:1]. Product properties: white solid, yield 82%. 1 H NMR (300MHz, CDCl 3 )δ0.87(t, 3H, J=6.3Hz), 1.25(s, 10H), 1.49(s, 2H), 3.22-3.44(m, 4H), 3.72(dd, 1H, J=6.6, 11.4Hz ), 3.83(t, 1H, J=7.2Hz), 3.98(d, 1H, J=6.9Hz), 4.54-4.79(m, 5H), 5.12(d, 1H, J=11.4Hz), 7.25-7.44 (m, 15H); 13 C NMR (75MHz, CDCl 3 )δ14.4, 23.0, 27.1, 27.7, 29.5, 29.7, 32.1, 47.4, 48.1, 72.4, 74.0, 74.5, 76.6, 79.7, 82.4, 128.0, 128.1, 128.2128.3, 128.6, 128.7, 128.8, 138.31, 138.1 , 138.4, 168.8.Anal.Calcd for C 34 h 43 NO 4 : C, 77.09; H, 8.18; N, 2.64; Found: C, 76.86; H, 7.92; N, 2.65; + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com