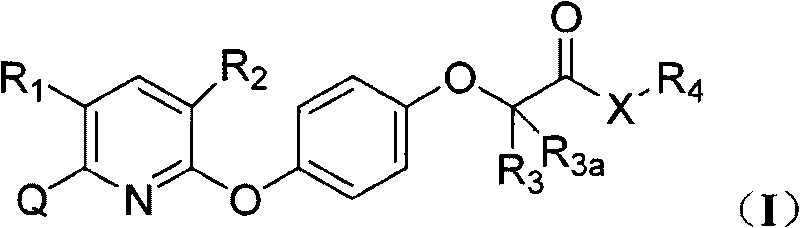
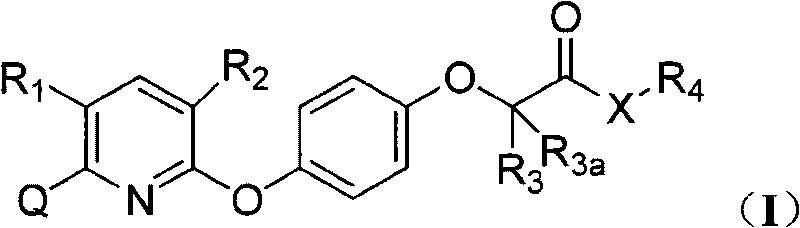
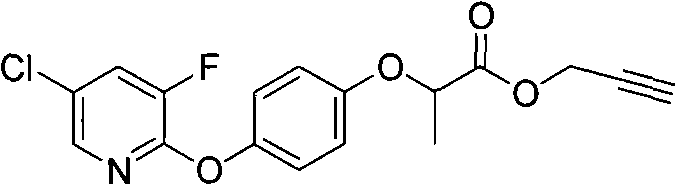
Pyridyloxy phenoxyalkanoic acids compound and application
A technology of pyridyloxyphenoxycarboxylic acid and compound, which is applied in the field of pyridyloxyphenoxycarboxylic acid compounds and applications, and can solve the problems of no bactericidal activity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0139] Example 1 (compound 1)
[0140] 1.1 Preparation of 2,3-dichloro-N-methoxypyridine-5-carboxamide
[0141]
[0142] 2.0 g of substituted pyridine acid chloride and 0.49 g of methoxylamine were stirred in 50 ml of toluene at 35-40°C for 8 hours, heated under reduced pressure to remove the toluene, added water and ethyl acetate, separated the organic layer, and washed with water and brine , dried and concentrated. The residue was purified by silica gel column chromatography to obtain 2.05 g of the target product with a yield of 94%.
[0143] 1.2 Preparation of intermediates
[0144]
[0145] 2,3-dichloro-N-methoxypyridine-5-carboxamide, 0.83 g of 2-(4-hydroxyphenoxy) propionic acid, 0.95 g of potassium carbonate, added to a 100 ml single-necked bottle, and 40 ml N,N-Dimethylformamide. Heat and stir at 80-90°C for four hours. After the reaction is complete, pour it into 100 ml of water, adjust the pH to about 2 with dilute hydrochloric acid, and extract with ethyl...
example 2
[0149] Example 2 (compound 52)
[0150]
[0151] 5.0 grams of 2,3-dichloro-5-cyanopyridine, 5.2 grams of 2-(4-hydroxyphenoxy)propionic acid, and 6 grams of potassium carbonate were added to a 250-milliliter single-necked bottle, and 80 milliliters of N,N -dimethylformamide. Heat and stir at 80-90°C for four hours. After the reaction is complete, adjust the pH to about 2 with dilute hydrochloric acid, and extract with ethyl acetate. Washed with saturated brine and concentrated to obtain 8.48 g of product. Yield 92%. 90% purity.
example 3
[0152] Example 3 (compound 46)
[0153]
[0154] 0.4 g of substituted phenoxypropionyl chloride and 0.1 g of ethylene glycol monomethyl ether were stirred in 50 ml of toluene at 35-40°C for 8 hours, heated under reduced pressure to remove toluene, added water and ethyl acetate, separated the organic layer, and Washed with water and brine, dried and concentrated. The residue was purified by silica gel column chromatography to obtain 0.2 g of the desired compound, melting at 130-132°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com