Medicine carrying polymer micelle and preparation method thereof
A drug-loaded polymer and micelle technology, which is used in pharmaceutical formulations, drug delivery, medical preparations with inactive ingredients, etc., can solve the problems of large particle size and large particle dispersion coefficient, and achieve good particle performance and micelles. The effect of stable state and ingestion avoidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
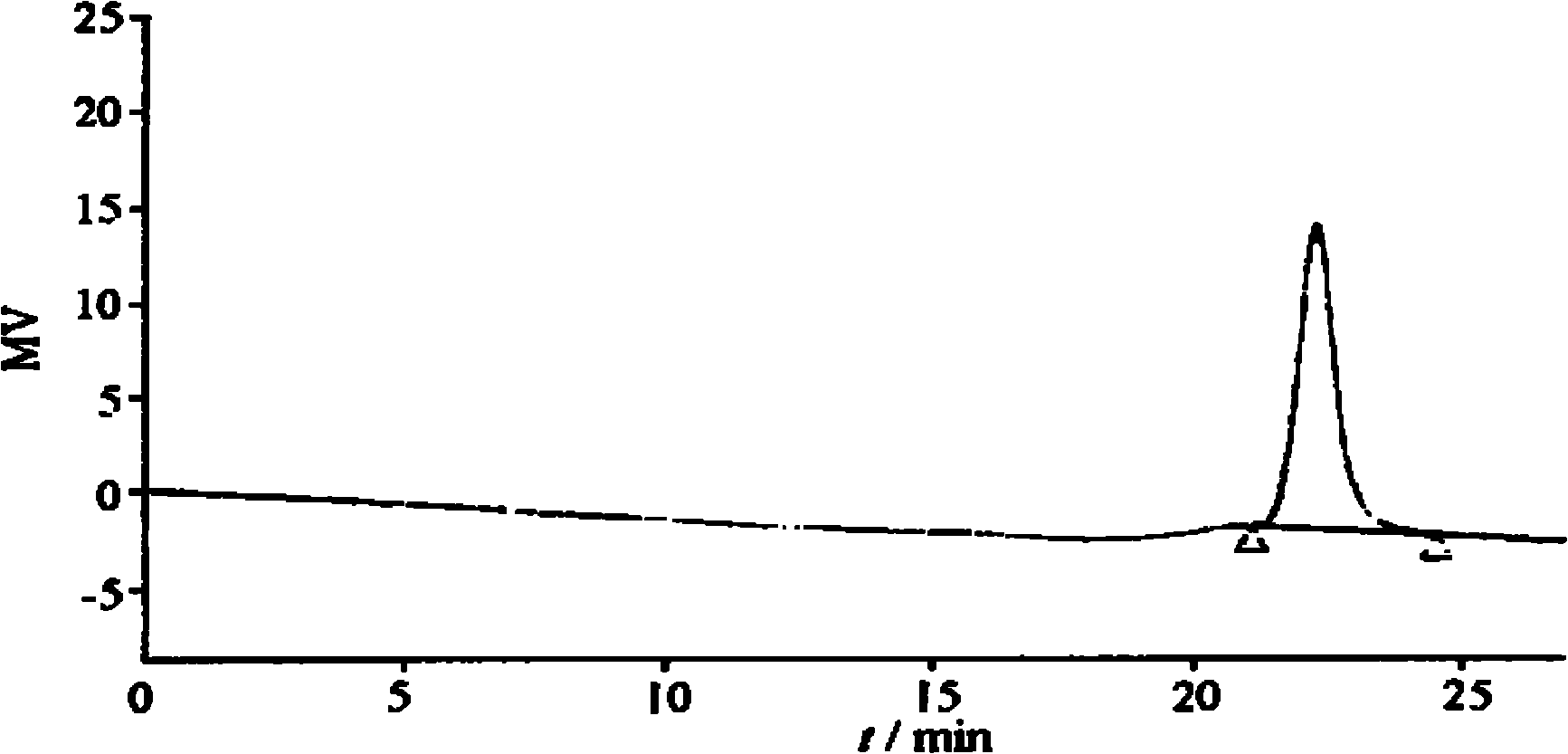
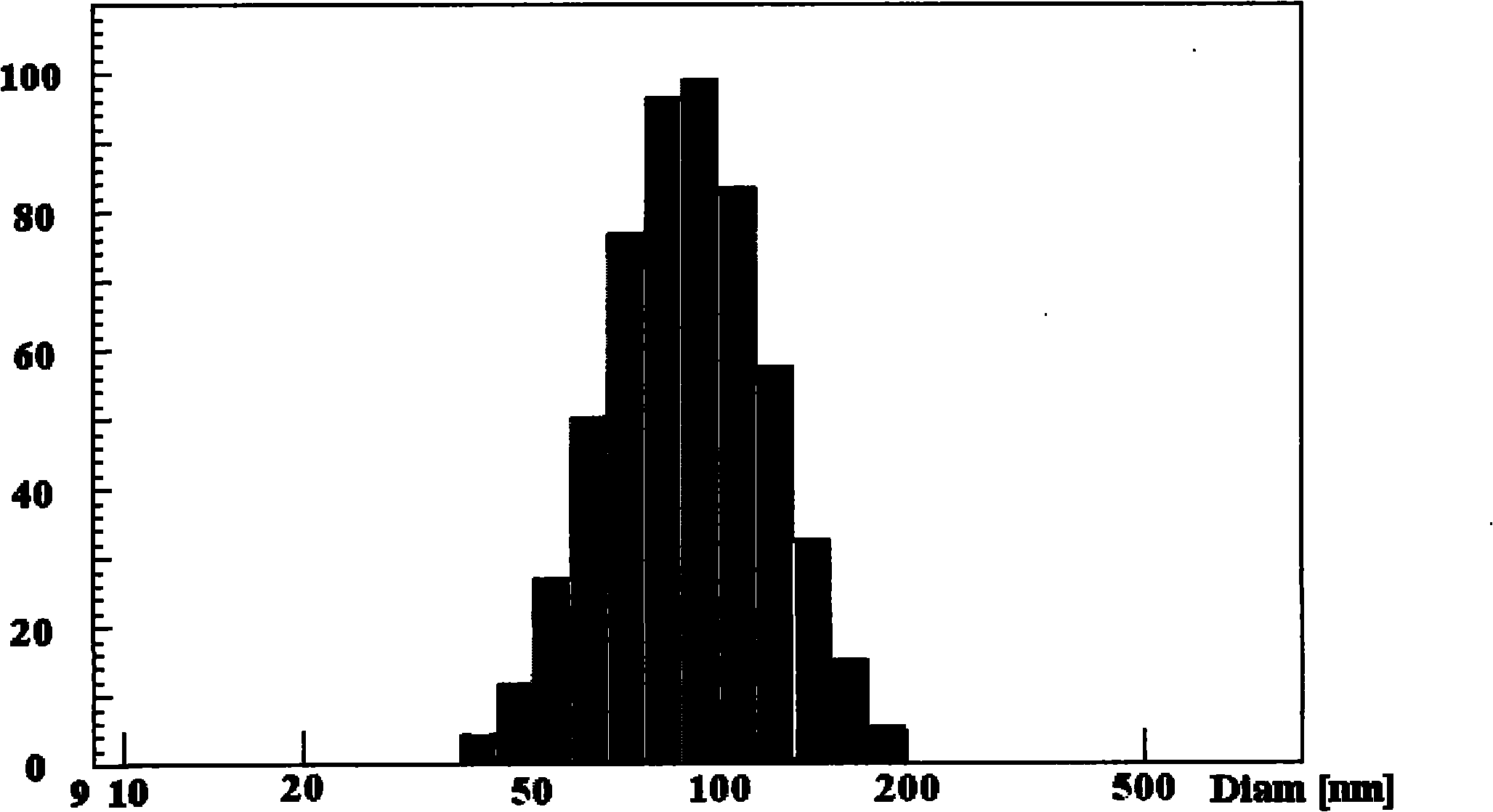
Examples
Embodiment 1
[0040] The synthesis of embodiment 1 monomethyl polyethylene glycol polylactic acid block copolymer
[0041] The lactide was recrystallized twice with ethyl acetate, and then dried under reduced pressure for 24 h. Monomethyl polyethylene glycol (MPEG, weight average molecular weight 5000) was dried under reduced pressure at 60° C. for 48 h. Weigh 2g of MPEG, 12g of lactide, and 0.03g of stannous octoate (catalyst) into a round-bottomed flask, add 10ml of toluene, heat up to 60°C, dissolve the reactant, then distill under reduced pressure to remove the toluene, leaving Down the white crystals. Use an oil pump to vacuum the bottle, then seal the flask, adjust the temperature of the oil bath to 150°C, and react for 40 hours. After the end, dissolve the product with 10ml of dichloromethane, and then add 40ml of ether to precipitate it. Repeat twice. , Dry under reduced pressure at 40°C for 40h to obtain the product, weighing 10.2g.
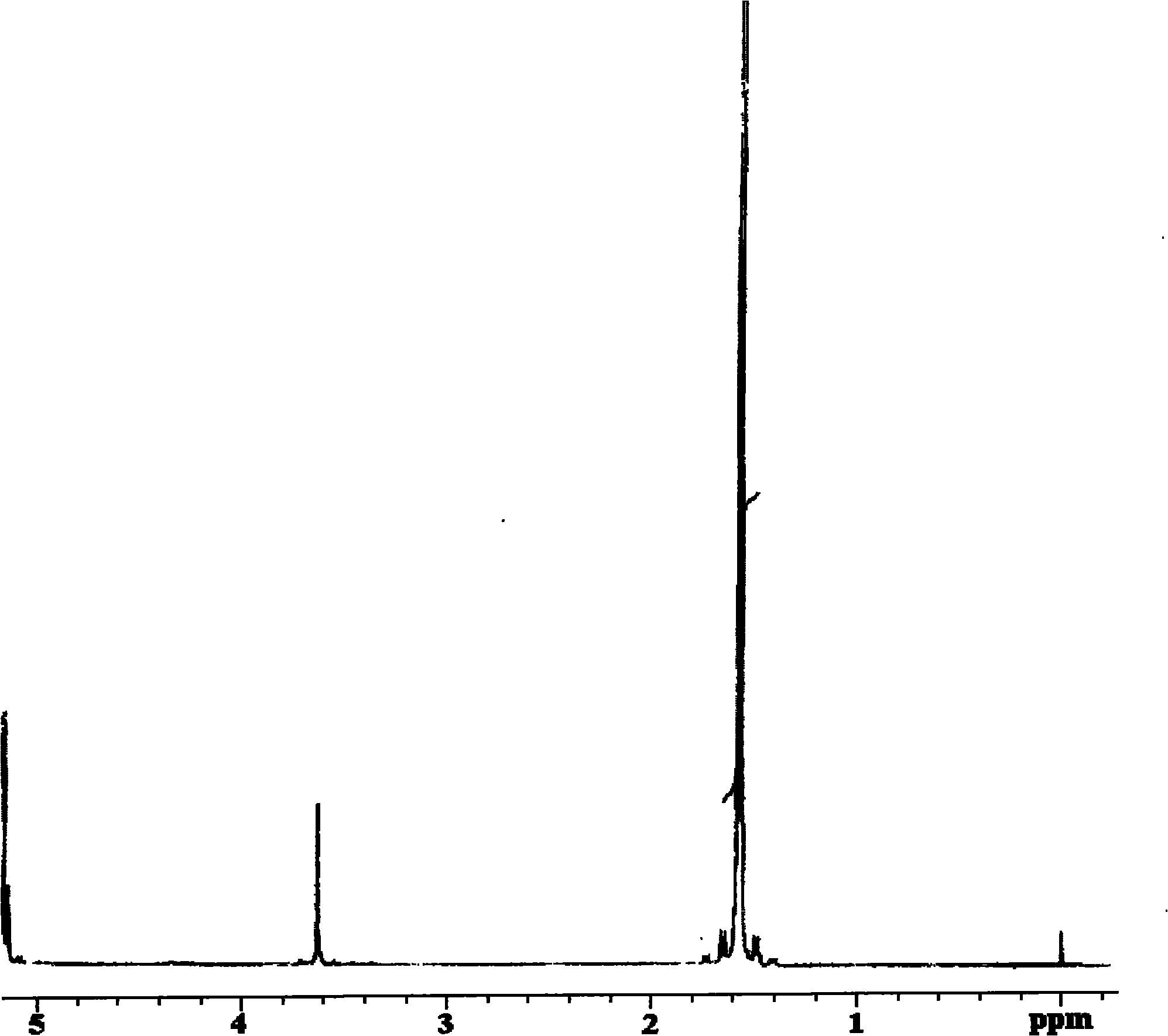
[0042] HNMR hydrogen spectrum was used to de...
Embodiment 2
[0043] The synthesis of embodiment 2 monomethyl polyethylene glycol polylactic acid block copolymers
[0044] The lactide was recrystallized twice with ethyl acetate, and then dried under reduced pressure for 24 h. Monomethyl polyethylene glycol (MPEG, weight average molecular weight 5000) was dried under reduced pressure at 60° C. for 48 h. Weigh 2.5g of MPEG, 10g of lactide, and 0.6g of stannous octoate (catalyst) into a round-bottomed flask, add 10ml of toluene, heat up to 60°C, dissolve the reactants, and then distill under reduced pressure to remove the toluene. White crystals remained. Use an oil pump to vacuum the bottle, then seal the flask, adjust the temperature of the oil bath to 120°C, and react for 50 hours. After the end, dissolve the product with 10ml of dichloromethane, and then add 40ml of ether to precipitate it. Repeat twice. , Dry under reduced pressure at 40°C for 40h to obtain the product, weighing 9.2g.
[0045] The block structure was determined by H...
Embodiment 3
[0046] The synthesis of embodiment 3 monomethyl polyethylene glycol polylactic acid block copolymers
[0047] The lactide was recrystallized twice with ethyl acetate, and then dried under reduced pressure for 24 h. Monomethyl polyethylene glycol (MPEG, weight average molecular weight 5000) was dried under reduced pressure at 60° C. for 48 h. Weigh 2g of MPEG, 16g of lactide, and 0.04g of stannous octoate (catalyst) into a round-bottomed flask, add 10ml of toluene, heat up to 60°C, dissolve the reactant, then distill under reduced pressure to remove the toluene, leaving Down the white crystals. Use an oil pump to vacuum the bottle, then seal the flask, adjust the temperature of the oil bath to 140°C, and react for 30 hours. After the end, dissolve the product with 10ml of dichloromethane, and then add 40ml of ether to precipitate it. Repeat twice. , Dry under reduced pressure at 40°C for 40h to obtain the product, weighing 15.2g.
[0048] The block structure was determined b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com