Method for preparing p-bromophenylacetone by biomimetic catalytic oxidation of p-bromoethylbenzene
A technology for the oxidation of p-bromoacetophenone and oxygen, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, and can solve the problem of increased equipment investment and operating costs, increased costs, increased operating costs and production costs and other issues to achieve the effect of reducing energy consumption and operating costs, reducing energy consumption and costs, and saving energy and resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
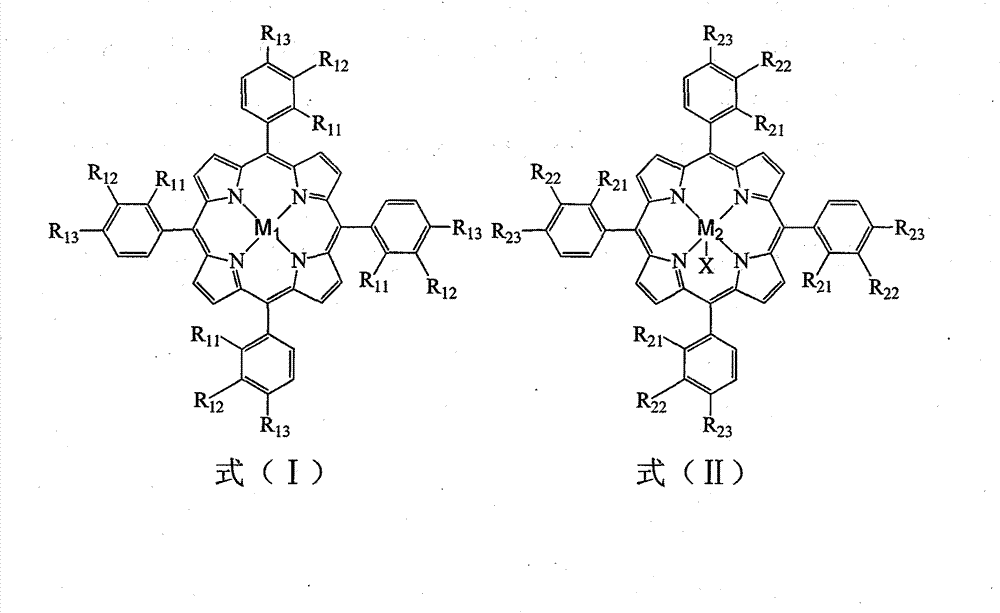
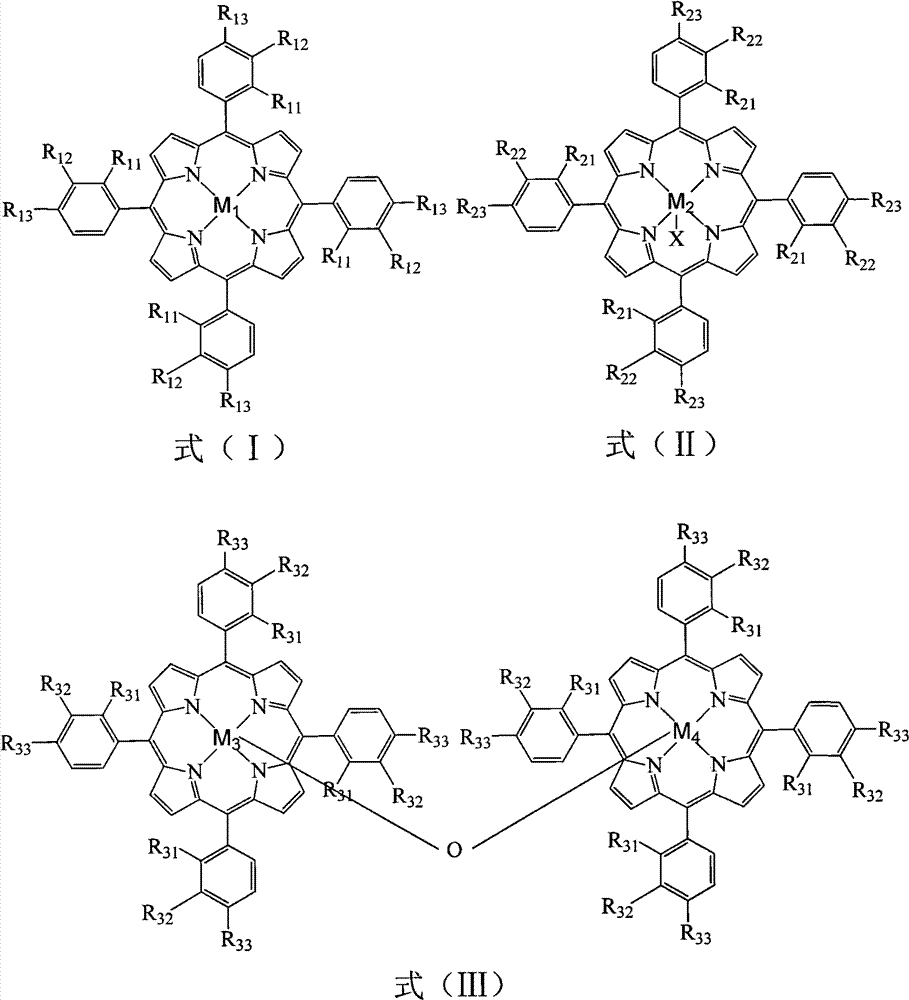
[0035] In a 100mL three-necked flask, add 18.531g p-bromoethylbenzene, 1ppm (0.07mg) tetraphenyliron porphyrin (i.e. R in formula (I) 11 for H, R 12 for H, R 13 for H, M 1 Fe), 10ppm (0.86mg) four-(p-chlorophenyl) cobalt porphyrin (that is, R in the formula (I) 11 for H, R 12 for H, R 13 for Cl, M 1 For Co), oxygen was introduced at a flow rate of 40 mL / min, the reaction was initiated at 150 °C, and the reaction was carried out at 100 °C for 8 h. The reacted mixture was frozen, centrifugally filtered, and then recrystallized with ethanol to obtain p-bromoacetophenone. The conversion rate of p-bromoethylbenzene was 79.8%, the yield of p-bromoacetophenone was 72.4%, and the purity was 99.5%.
Embodiment 2
[0037] In a 100mL three-necked flask, add 18.491g p-bromoethylbenzene, 5ppm (0.38mg) tetra-phenylmanganese porphyrin chloride (that is, R in formula (II) 21 for H, R 22 for H, R 23 for H, M 2 is Mn, X is Cl), 5ppm (0.44mg) tetrakis-(o-methoxyphenyl) cobalt porphyrin chloride (that is, R in the formula (II) 21 for OCH 3 , R 22 for H, R 23 for H, M 2 is Co, X is Cl), oxygen was introduced at a flow rate of 40 mL / min, the reaction was initiated at 150 °C, and the reaction was carried out at 100 °C for 10 h. The reacted mixture was frozen, centrifugally filtered, and then recrystallized with ethanol to obtain p-bromoacetophenone. The conversion rate of p-bromoethylbenzene was 83.8%, the yield of p-bromoacetophenone was 76.5%, and the purity was 99.3%.
Embodiment 3
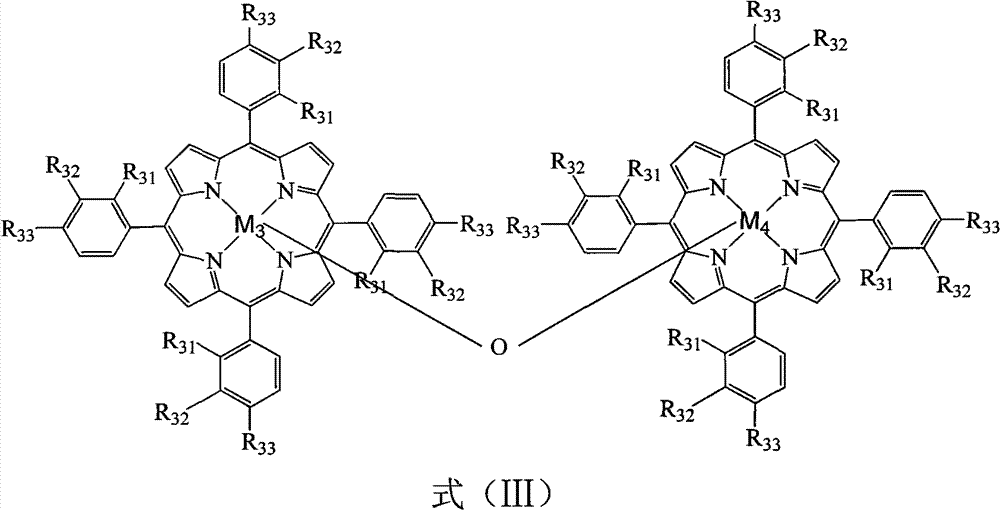
[0039] In a 100mL three-necked flask, add 18.502g p-bromoethylbenzene, 1ppm (0.15mg) μ-oxygen-binuclear tetra-phenyliron porphyrin (that is, R in formula (III) 31 for H, R 32 for H, R 33 for H, M 3 , M 4 Fe), 5ppm (0.88mg) μ-oxygen-binuclear four-(p-chlorophenyl) cobalt porphyrin (that is, R in formula (III) 31 for H, R 32 for H, R 33 for Cl, M 3 , M 4 For Co), oxygen was introduced at a flow rate of 50 mL / min, the reaction was initiated at 150 °C, and the reaction was carried out at 90 °C for 10 h. The reacted mixture was frozen, centrifugally filtered, and then recrystallized with ethanol to obtain p-bromoacetophenone. The conversion rate of p-bromoethylbenzene was 71.2%, the yield of p-bromoacetophenone was 64.3%, and the purity was 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com