1-halogenated-1, 4-diene nitrile and synthetic method thereof
A kind of diene nitrile and olefin technology, applied in the field of 1-halogenated-1,4-diene nitrile and its synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
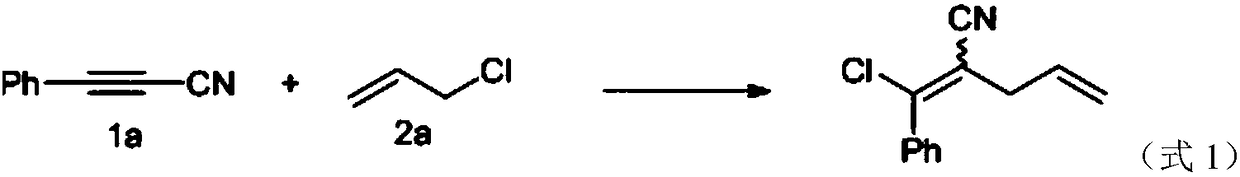
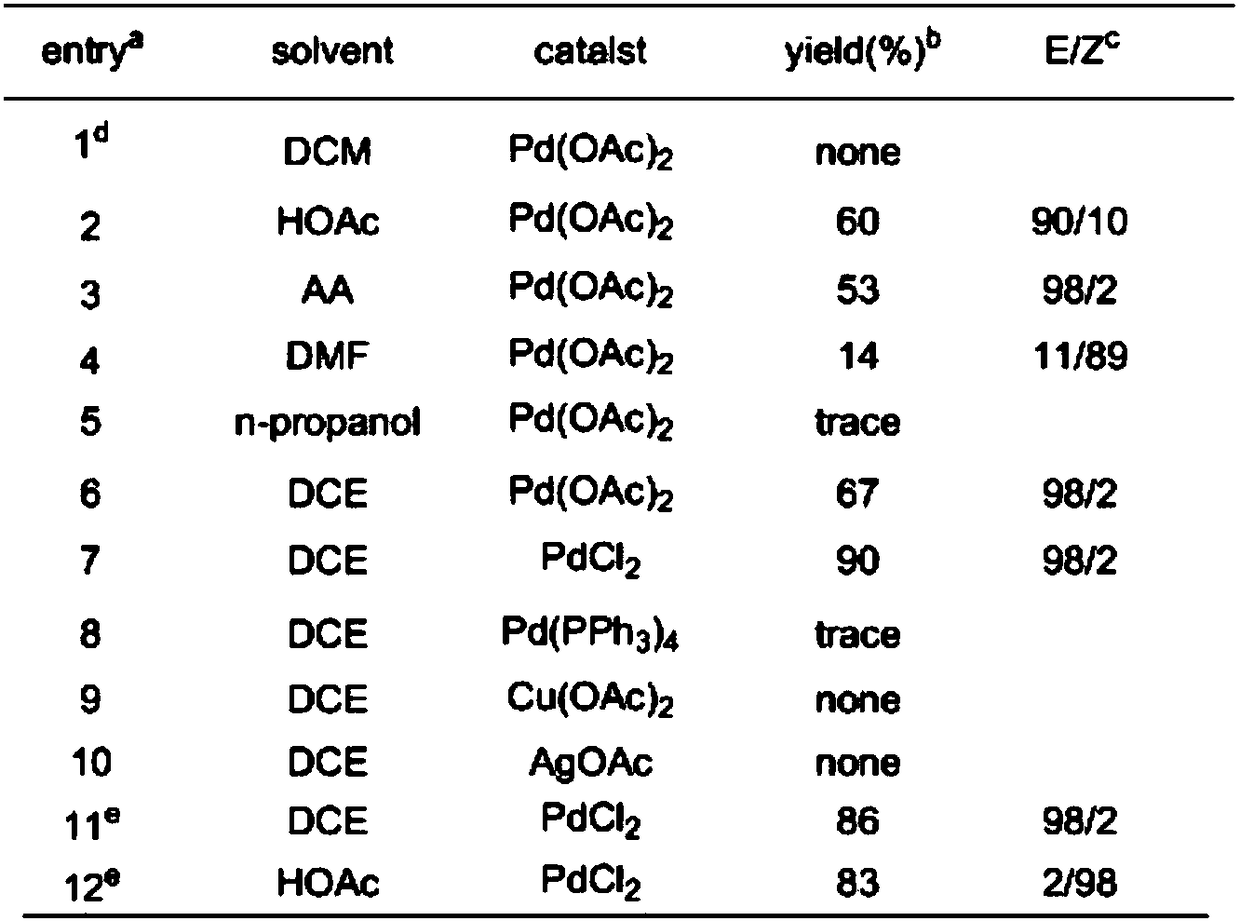
[0022] With phenylacetylene nitrile (1a) and 3-chloroprop-1-alkene (2a) as reactants, palladium compound (palladium acetate, palladium chloride) as catalyst, dichloroethane, acetic acid, acetic anhydride or dimethyl 1-halogeno-1,4-dienenitrile was obtained by addition reaction at a reaction temperature of 70°C using methyl formamide as a solvent; among them, aromatic cyanoalkyne, 3-chloroprop-1-alkene The reaction ratio of palladium compound and solvent is 0.25mmoL: 0.5mmoL: 0.0125mmoL: 2mL; the specific reaction chemical formula is as shown in formula 1, under the constant situation of reactant, change solvent kind and catalyst kind, obtain different chromatographic products The experimental data of the rate and the highly selective Z / E structure are shown in Table 1.
[0023]
[0024] The productive rate of table 1. embodiment 1 same reactant different solvent different catalysts and high selectivity Z / E formula structural data
[0025]
[0026] a Reaction conditions...
Embodiment 2
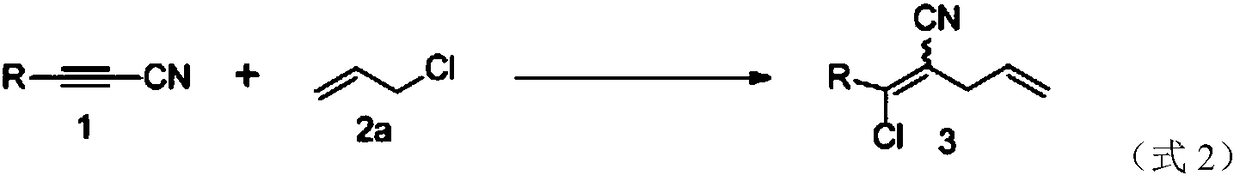
[0028] With aryl alkyne (1) and 3-chloroprop-1-alkene (2a) as reactant, add lithium chloride, take palladium compound (palladium acetate, palladium chloride) as catalyst, take acetic acid as solvent, in the reaction A highly selective Z-stereostructure 1-chloro-1,4-dienenitrile compound is obtained through an addition reaction at a temperature of 35°C; among them, alkylcyanoalkynes, 3-chloroprop-1-alkenes, The reaction ratio of lithium chloride, palladium compound and acetic acid is 0.5mmoL: 1.0mmoL: 1.0mmoL: 0.025mmoL: 2mL; the specific reaction chemical formula is shown in formula 2, and different kinds of alkylcyanoalkynes obtain different chromatographic yields. For the experimental data, see Table 2 for details.
Embodiment 3
[0030] With aryl alkyne (1) and 3-chloroprop-1-alkene (2a) as reactant, with palladium compound (palladium acetate, palladium chloride) as catalyst, with dichloroethane as solvent, at the reaction temperature of 1-Chloro-1,4-dienenitrile compounds with highly selective E-stereostructure are obtained by addition reaction at 70°C; among them, alkylcyanoalkynes, 3-chloroprop-1-alkenes, chlorinated The reaction ratio of lithium, palladium compound and acetic acid is 0.5mmoL: 1.0mmoL: 0.025mmoL: 2mL; the specific reaction chemical formula is shown in formula 2, different kinds of alkylcyanoalkynes get different chromatographic yield experimental data, see table for details 2.
[0031]
[0032] Table 2. The productive rate and highly selective Z / E formula structural data of embodiment 2 and implementation 3 different alkyl cyanoalkyne species
[0033]
[0034] Method A: formation of Z. Reaction conditions: 1(0.5mmol), 2a(1.0mmol), LiCl(1.0mmol) and [Pd](0.025mmol) in 2mL of H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com