Preparation method of p-nitroacetophenone
A technology of p-nitroacetophenone and p-nitroethylbenzene, which is applied in the field of preparation of p-nitroacetophenone, can solve the problems such as difficulty in realizing scale-up industrial production, difficulty in scale-up to realize industrial production, and small amount of reaction substrates. , to achieve the effect of increasing yield, avoiding emission and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
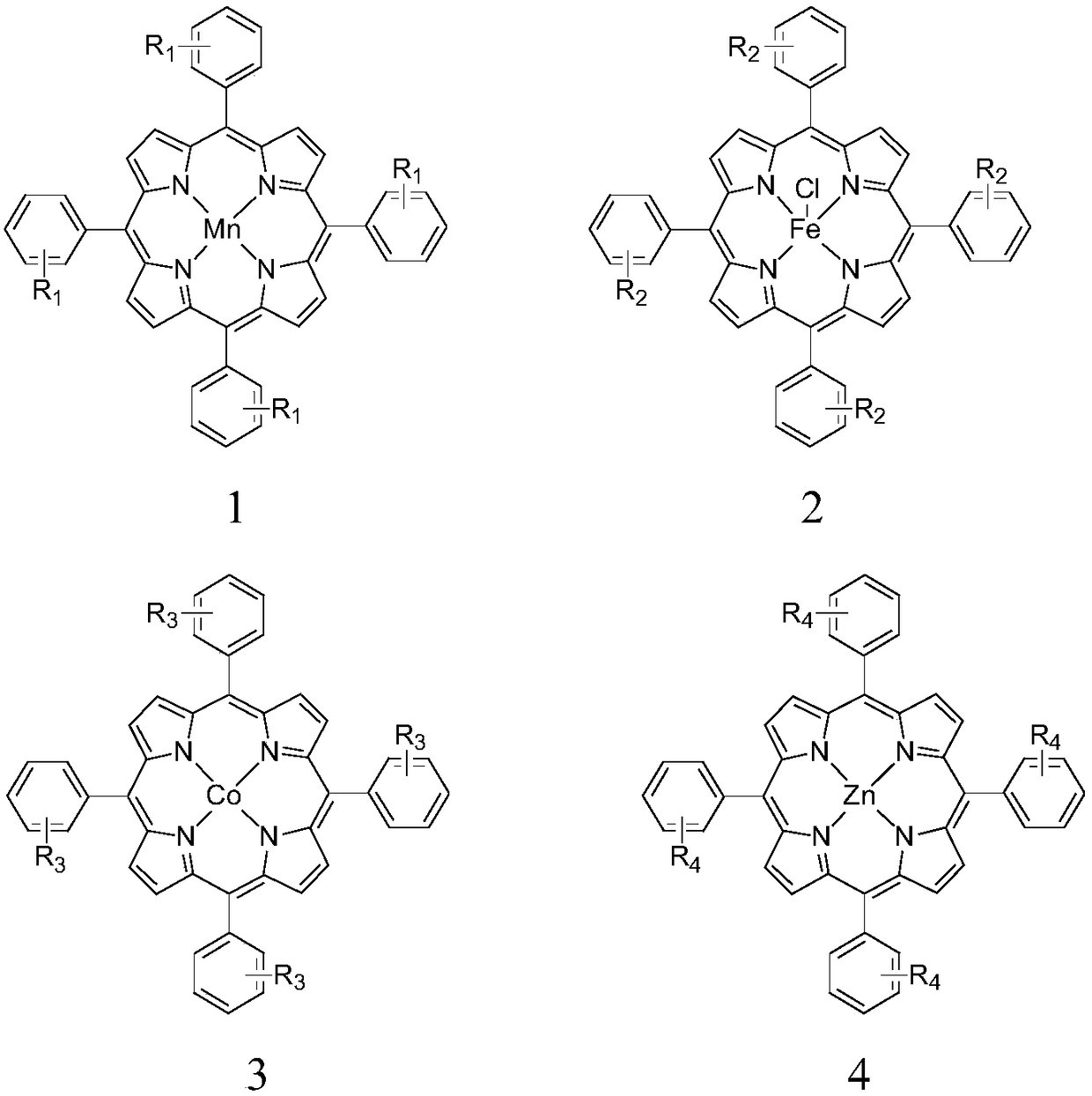
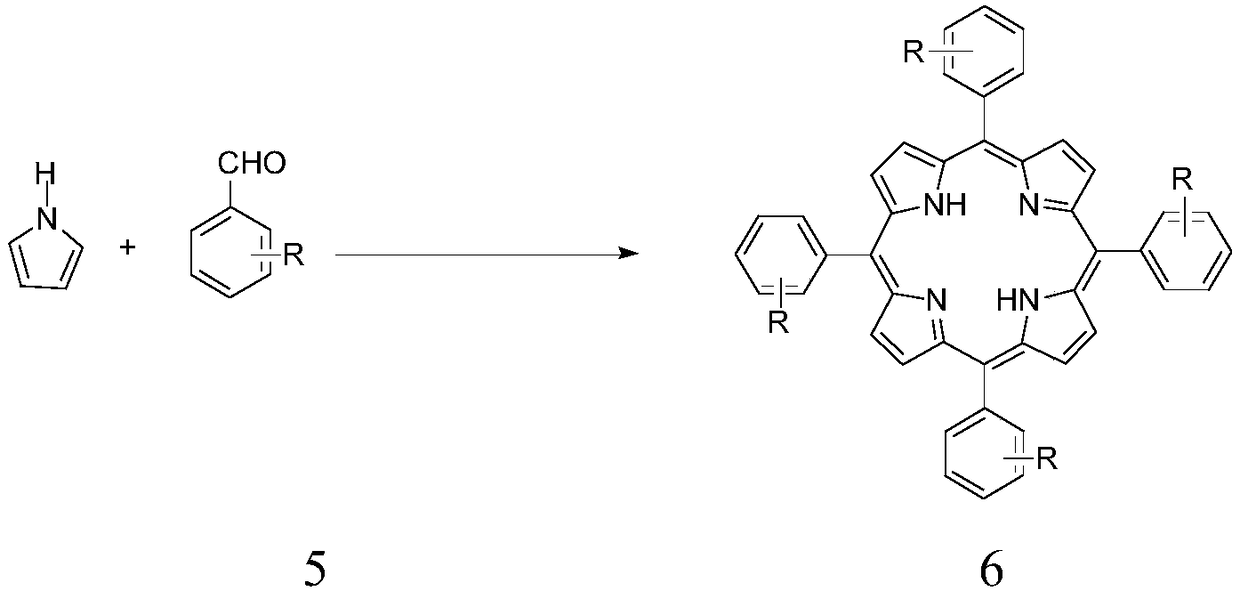
[0035] Metalloporphyrin catalyst of the present invention is specifically prepared according to the following method:
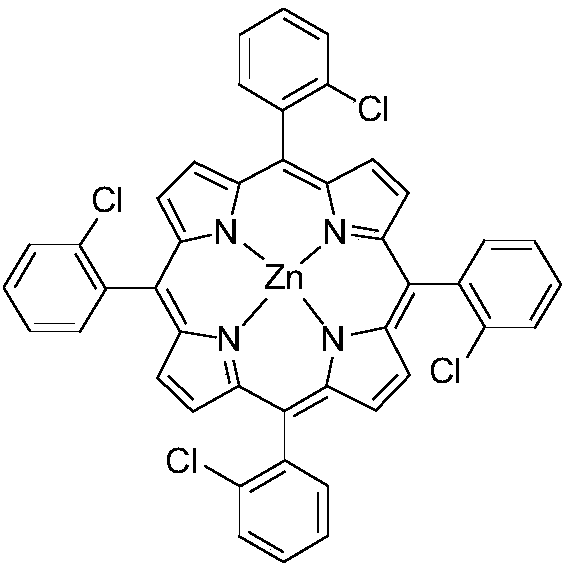
[0036] T(o-Cl)PPZn (structure as follows):
[0037]
[0038] Take a 100mL three-neck flask, pump nitrogen three times, then add 20mmol (2.8112g) o-chlorobenzaldehyde and 20mmol (1.3418g) pyrrole, 20mL propionic acid and 20mL acetic acid as solvent, 5μL trifluoroacetic acid as catalyst, 4mL nitrobenzene As an oxidizing agent, react at 140°C for 4 h, cool the reaction system to room temperature, add 100 mL of methanol, then filter, wash the filter cake with methanol, and recrystallize the filter cake with methanol to obtain ligand 204.3 mg T(o-Cl )PP, the yield is 1.4%, then take 0.13mmol (100mg) of the above-mentioned ligand T(o-Cl)PP in a 100mL three-necked round-bottomed flask, add 1.3mmol (238.5mg) zinc acetate, and replace nitrogen three times, Add the solvent DMF, react at 140°C for 12h, distill off the solvent with an oil pump under reduced pressure,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com