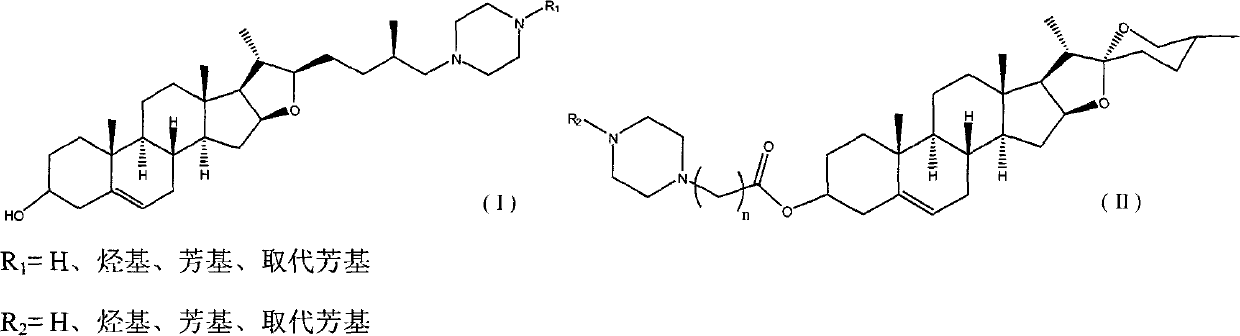
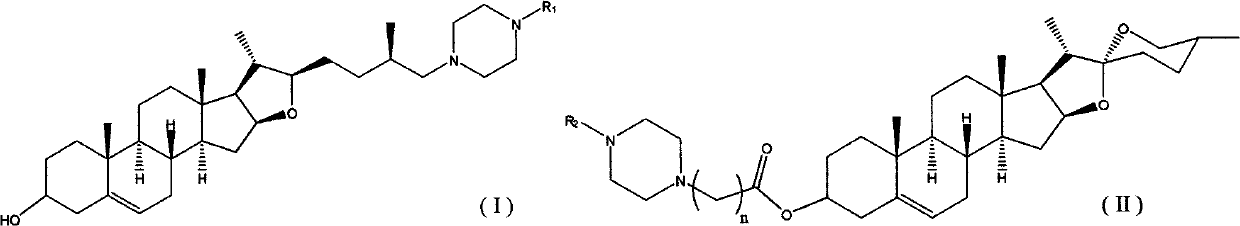
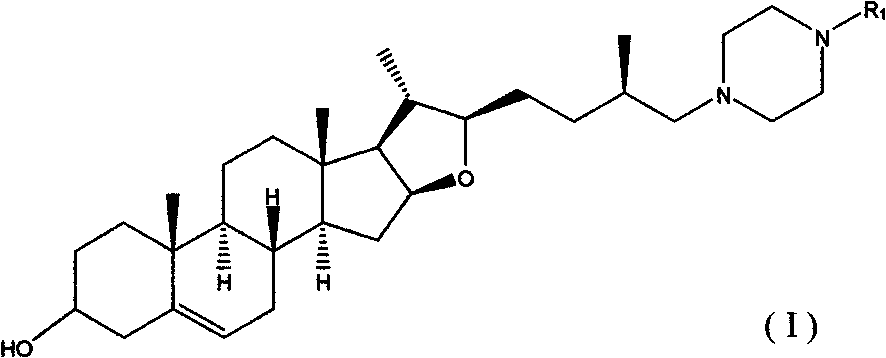
Diosgenin piperazine derivatives and preparation method thereof
A technology of diosgenin and aglycone piperazine, which is applied in the directions of drug combination, steroids, antitumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0050] The specific embodiment: enumerate typical compound of the present invention below in conjunction with example
example 1
[0051] The synthesis of example 1 diosgenin loss F ring 27-piperazine compound (1)
[0052]
[0053] 1, the synthesis (I) of diosgenin 3-Ac: get diosgenin (2g, 4.8mmol), acetic anhydride (4ml, 40.2mmol) in pyridine (8ml), after stirring in ice bath for 30 minutes, be heated to 60°C, react for 3 hours. After the reaction was completed, it was cooled to room temperature, and then the reaction solution was poured into ice water (500ml) and stirred for 1 hour, then filtered, and the solid was washed with ice water (50ml*5) and vacuum-dried to obtain a white solid.
[0054]
[0055] 2. Synthesis of diosgenin 3-Ac missing F ring (II): Dissolve compound I (1.5 g) in dichloromethane (8 ml), slowly add glacial acetic acid (20 ml) dropwise at room temperature, and stir for 10 minutes , added sodium cyanoborocyanide (1.060g, 16.51mmol) in batches, after reacting at room temperature for 5 hours, added water (15ml) and stirred for 10 minutes, then extracted with dichloromethane, and t...
example 2
[0061] Example 2 Synthesis of 1-diosgenin lost F ring-4-substituted-piperazine compound (2)
[0062]
[0063] Compound 1 (175mg, 0.35mmol) and benzyl bromide (140mg, 0.82mmol) were dissolved in acetonitrile (15ml), and reacted at 60°C for 5 hours. After the solvent was evaporated under reduced pressure, it was dissolved with dichloromethane (30ml), and the solution was washed twice with saturated sodium bicarbonate solution, once with saturated brine, dried over anhydrous sodium sulfate, and the insoluble matter was filtered out, and the filtrate was spin-dried under reduced pressure, leaving The product was purified by column chromatography to obtain 1-diosgenin lost F ring-4-substituted-piperazine compound (147mg), mp220-221°C, yield: 60.4%.
[0064] 1 HNMR (CDCl3, 400MHz): 7.52-7.49 (dd, J = 13.6HZ, 2); 7.47-7.38 (m, 3H); 5.35-5.34 (t, J = 4.4HZ, 1H); 3.93 (S, 2H)
[0065] 13 CNMRCDCL3,100MHz):δ140.81,133.77,130.78,129.33,128.87,121.34,89.77,83.26,71.66,64.83,63.58,61...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com