Compositions and methods for treating the vertebral column
A composition and drug technology, applied in the direction of drug combination, application, tissue regeneration, etc., can solve the problems of preventive treatment and prevention of secondary VCF unmet clinical needs, increasing morbidity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0108] The present invention provides a preparation method of a composition for promoting bone formation in a vertebral body and preventing or reducing the possibility of vertebral body compression fractures (including secondary vertebral fractures). In one embodiment, the method for preparing said composition comprises: providing a PDGF-containing solution, providing a biocompatible matrix and disposing said solution in said biocompatible matrix. Suitable PDGF solutions and biocompatible matrices for the combination correspond to those PDGF solutions and biocompatible matrices described above.
[0109] In some embodiments, the PDGF solution can be disposed in the biocompatible matrix by wetting the biocompatible matrix in the PDGF solution. In another embodiment, the PDGF solution can be disposed in the biocompatible matrix by injecting the PDGF solution into the biocompatible matrix. In some embodiments, injecting the PDGF solution can include disposing the PDGF solution in...
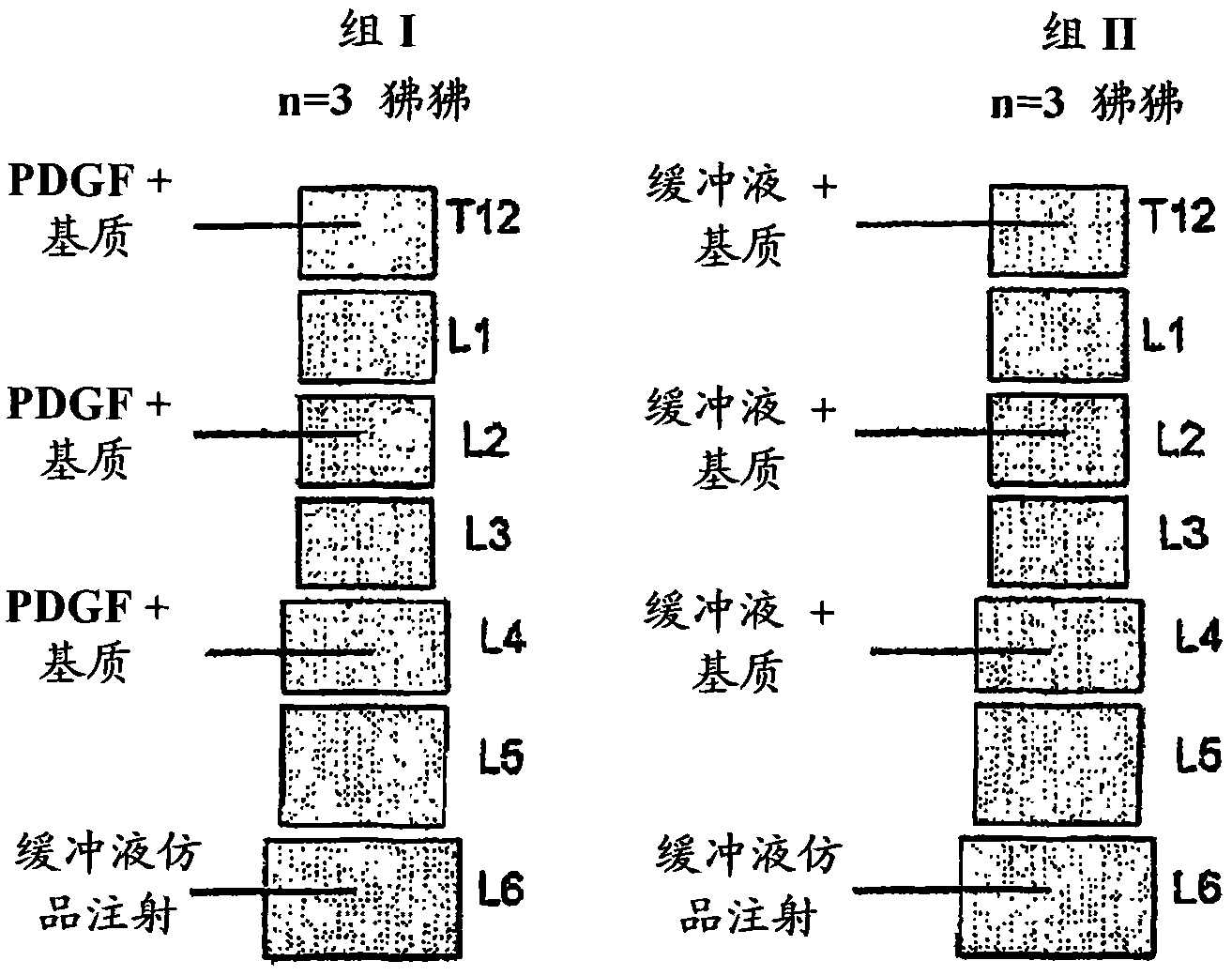
Embodiment 1
[0126] Preparation of compositions comprising PDGF solutions and biocompatible matrices
[0127] Compositions comprising PDGF solutions and biocompatible matrices were prepared as follows.
[0128] Obtain a pre-weighed batch of biocompatible matrix containing β-TCP and collagen. The β-TCP comprises β-TCP particles having an average diameter between about 100 μm and about 300 μm. The β-TCP particles were formulated with about 20% by weight soluble bovine type I collagen binder. The β-TCP / collagen biocompatible matrix is commercially available from Kensey Nash (Exton, Pennsylvania).
[0129] A solution containing rhPDGF-BB is obtained. A stock solution of rhPDGF-BB in sodium acetate buffer at a concentration of 10 mg / ml (ie, lot number QA2217) was purchased from Novartis Corporation. The rhPDGF-BB is produced by Chiron Corporation in the yeast expression system, and the rhPDGF-BB used in the products REGRANEX (Johnson & Johnson) and GEM 21S (BioMimetic Therapeutics) comes fr...
Embodiment 2
[0132] Methods of inhibiting secondary vertebral compression fractures
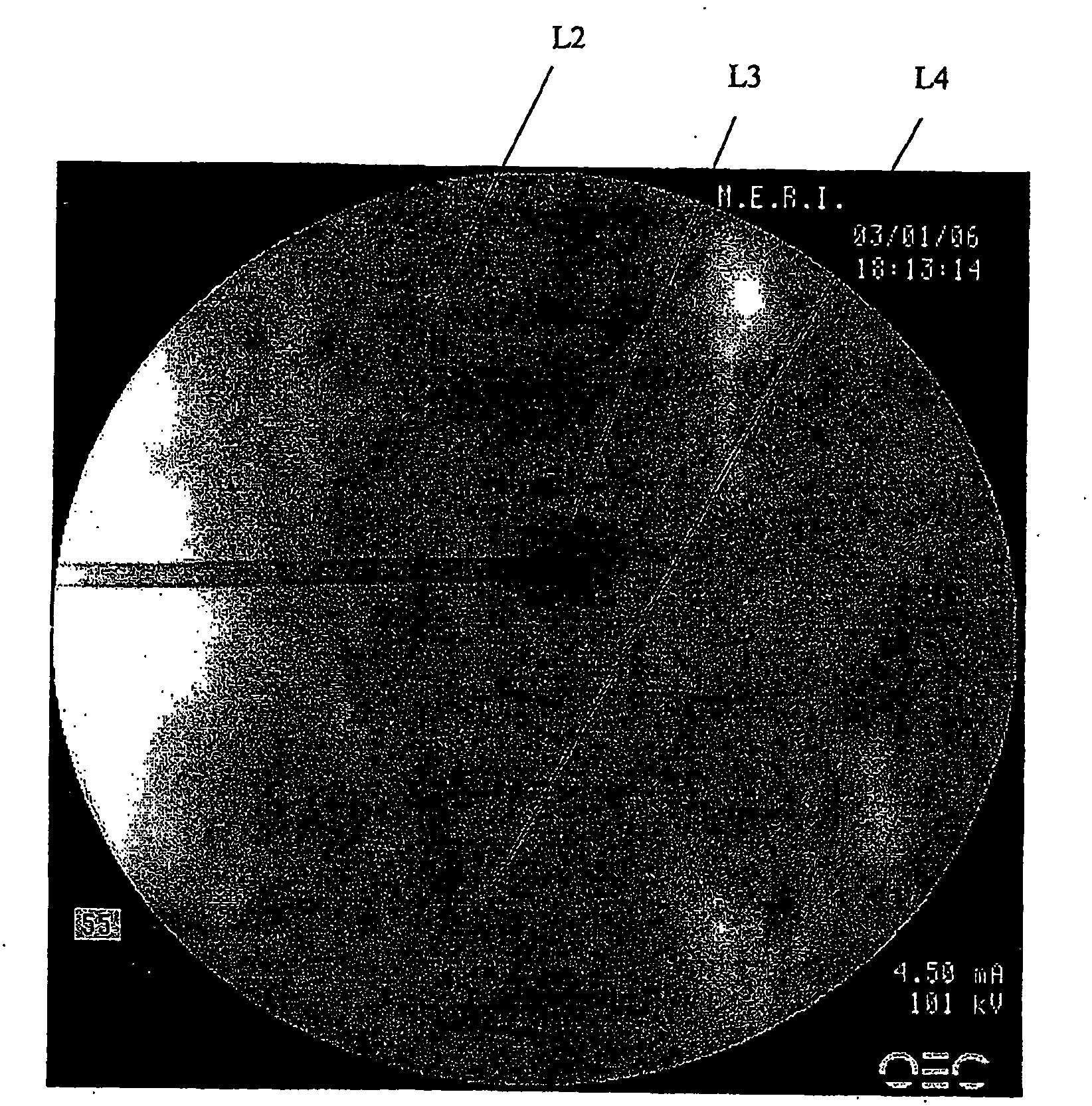
[0133] Experimental Design and Overview
[0134] This prospective randomized, controlled, single-centre clinical trial was to evaluate a composition comprising PDGF solution formulated in a tricalcium phosphate matrix for inhibiting high-risk vertebral bodies during vertebral compression fracture kyphoplasty (FFVB) Efficacy in secondary compression fractures. A comparison was made between vertebral bodies treated with the [beta]-tricalcium phosphate+rhPDGF-BB composition and untreated vertebral bodies. This study is a pilot clinical trial to support the evidence or principle that β-TCP+rh-PDGF-BB prevents or reduces the possibility of secondary vertebral compression fractures by increasing HVB bone formation.
[0135] This study was performed in a total of up to 10 patients requiring prophylactic treatment of HVB at the time of kyphoplasty. Potential patients were screened to determine if they met incl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com