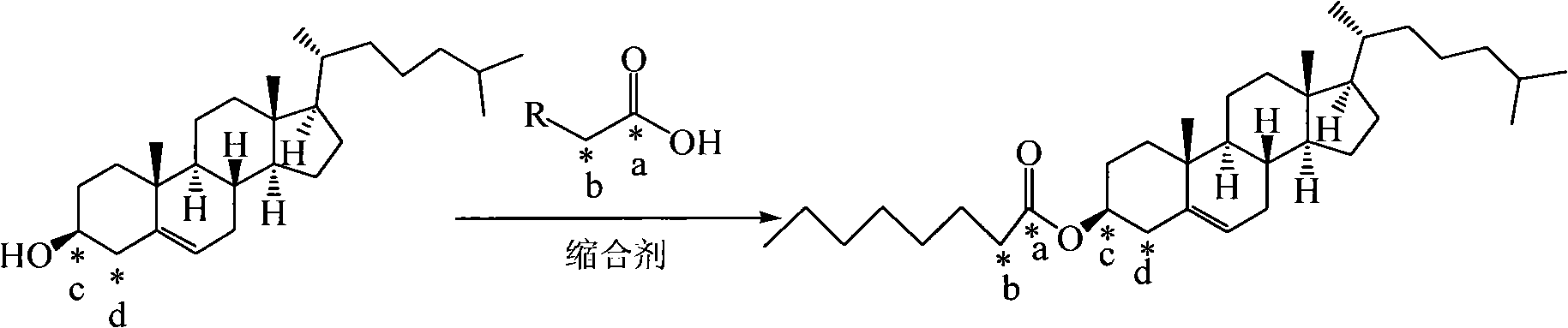
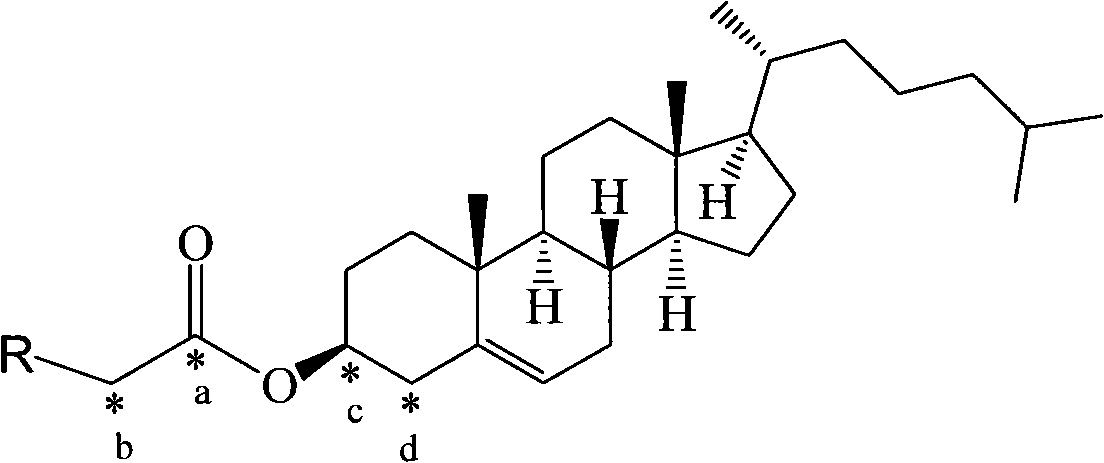
Method for synthesizing 13C-labeled cholesterol carboxylic ester
A technology of cholesteryl carboxylate and cholesteryl caprylate, which is applied in the field of synthesis of 13C-labeled cholesteryl carboxylate, and can solve problems such as the absence of cholesteryl caprylate reported in literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Cholesteryl caprylate-octanoyl-1- 13 Synthesis of C
[0036] Under nitrogen protection, cholesterol (0.002mol) and octanoic acid-1- 13 C (0.004mol) was dissolved in DMSO (55ml), and EEDQ (0.005mol) and HOBt (0.004mol) were added at 0°C. After the addition was complete, the reaction continued for 40 hours at 10°C. After the reaction solution was concentrated, it was purified by column chromatography. A white solid was obtained (yield 85%).
Embodiment 2
[0038] Cholesteryl Laurate-Lauroyl-1- 13 Synthesis of C
[0039] Under nitrogen protection, cholesterol (0.002mol) and lauric acid-1- 13 C (0.003mol) was dissolved in dichloroethane (45ml), and EEDQ (0.005mol) and DCC (0.004mol) were added at 0°C. After the addition was completed, the reaction was continued at 20°C for 36 hours. After the reaction solution was concentrated, it was passed through the column Chromatographic purification afforded a white solid (yield 82%).
Embodiment 3
[0041] Cholesteryl Palmitate-Palmitoyl-1- 13 Synthesis of C
[0042] Under nitrogen protection, cholesterol (0.002mol) and palmitic acid-1- 13 C (0.005mol) was dissolved in chloroform (50ml), and HOBt (0.005mol) and DCC (0.004mol) were added at 10°C. After the addition, the reaction continued at 30 for 24 hours. After the reaction solution was concentrated, it was purified by column chromatography to obtain White solid (85% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com