Biosynthesis method of isotope 13C labeling 4-oxyproline
A hydroxyproline and biosynthesis technology, applied in the field of 4-hydroxyproline synthesis, can solve the problems that 4-hydroxyproline is not suitable for the synthesis of 4-hydroxyproline, etc., achieving low cost and rapid effect , The effect of simple purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
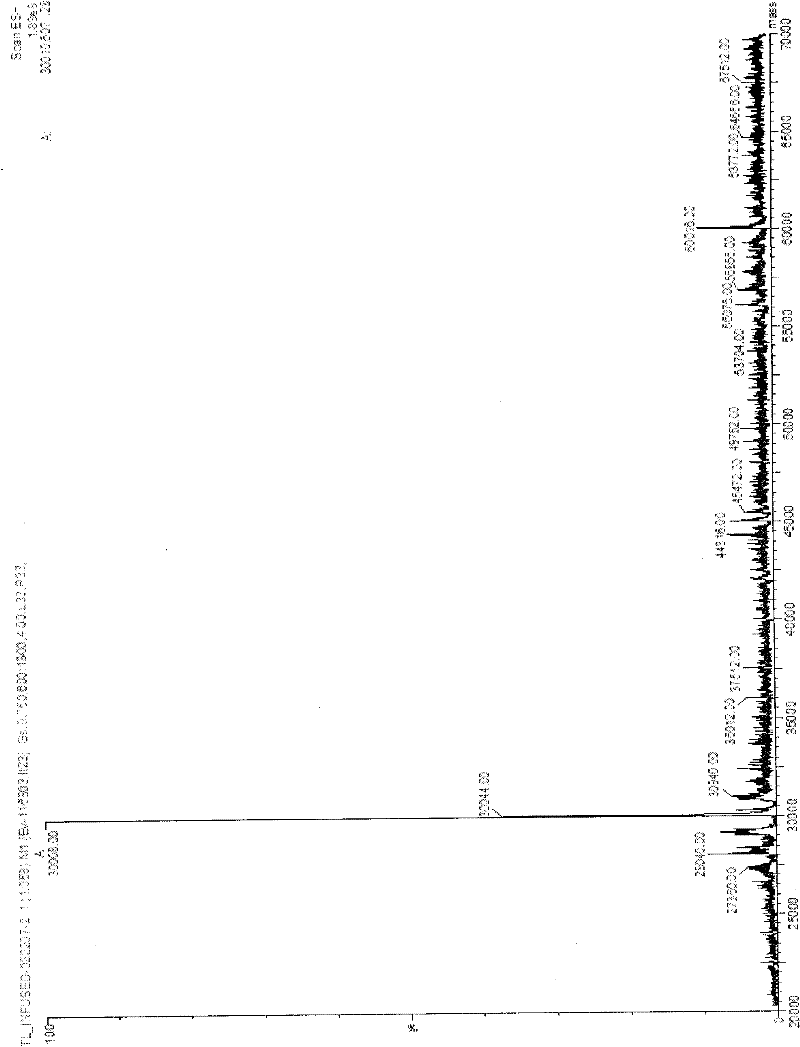
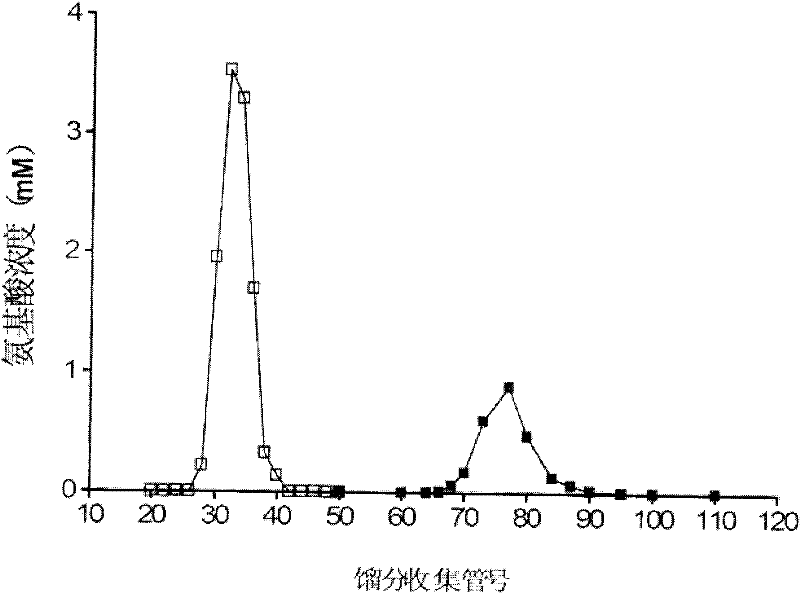
[0011] Specific implementation mode one: the isotope of this implementation mode 13 The biosynthetic method of the 4-hydroxyproline of C mark is carried out as follows: one, carry out codon optimization to the original sequence of the gene phy-1 of the 4-hydroxylase of coding L-proline in the cystic fungus , to obtain the optimized phy-1 gene; 2. The optimized phy-1 gene and the modified prokaryotic expression vector pET19 were subjected to double enzyme digestion with NdeI and BamHI, respectively, and the desired target fragment was recovered, and the prokaryotic expression vector pET19-pp was connected and constructed -histag-phy-1, and then RNA polymerase was inserted into Escherichia coli C41 on DE3 for high-efficiency protein expression and purification to obtain purified L-proline 4-hydroxylase; 3. Purified L- The 4-hydroxylase of proline performs an enzymatic synthesis reaction to generate a reaction product, which is then purified to obtain 13 C-labeled 4-hydroxyproli...
specific Embodiment approach 2
[0029] Specific embodiment two: the difference between this embodiment and specific embodiment one is that the enzyme digestion system in step two is as follows:
[0030] Ingredients Dosage
[0031] 10× buffer 10μL
[0032] 10U / μL NdeI 2μL
[0033] 10U / μL BamHI 2μL
[0034] 1 μg / μL optimized phy-1 gene / prokaryotic expression vector pET19 10 μL
[0035] Nuclease-free purified water 76 μL.
[0036] Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0037] Specific embodiment three: the difference between this embodiment and specific embodiment one is that the connection system in step two is as follows:
[0038] Ingredients Dosage
[0039] 10× buffer 1 μL
[0040] 100ng / μL optimized phy-1 gene after double enzyme digestion 1μL
[0041] 100ng / μL prokaryotic expression vector pET19 after double enzyme digestion 5μL
[0042] 10U / μL T4 ligase 1μL.
[0043] Other steps and parameters are the same as those in Embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com