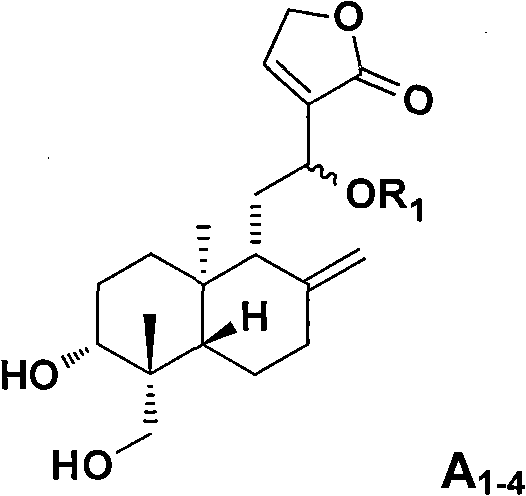
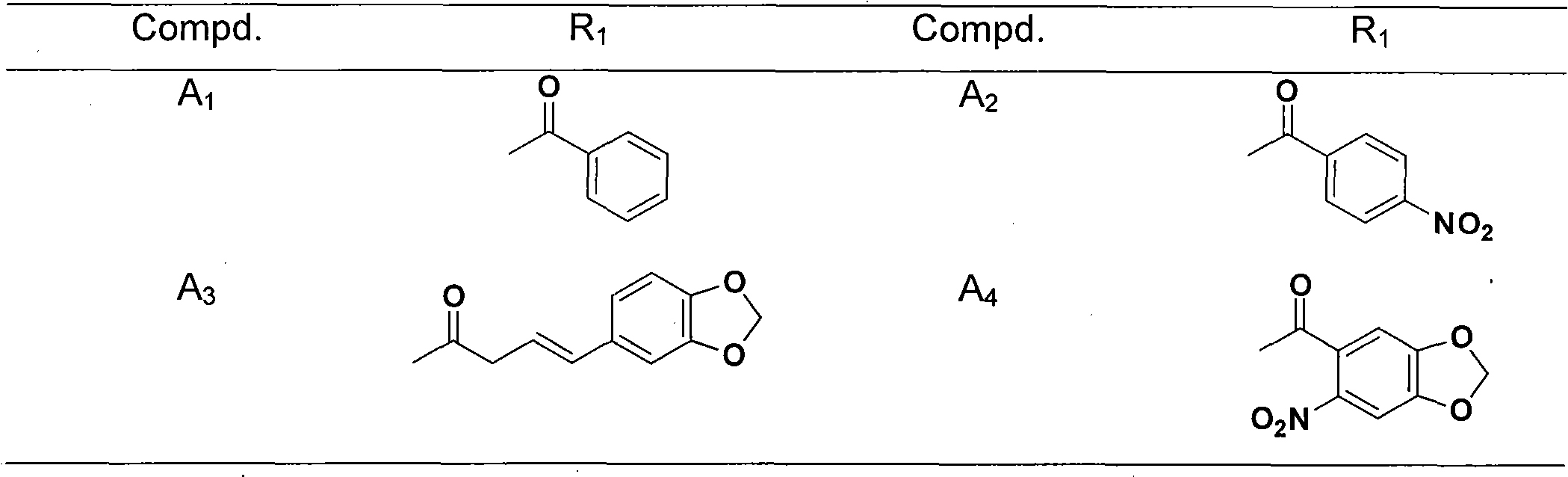
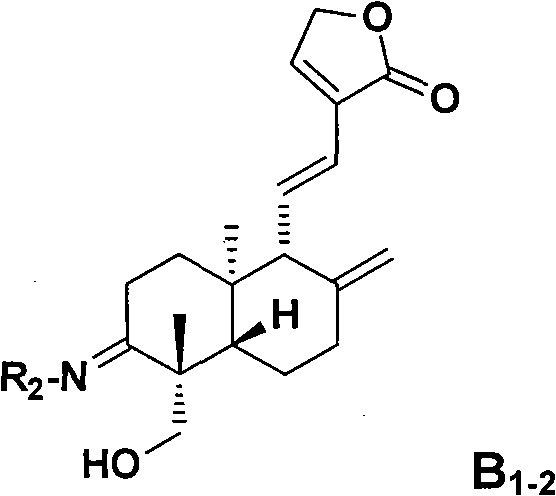
Applications of andrographolide derivatives in preparing anti-HIV medicines
A technology of andrographolide and deoxyandrographolide, applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of anti-HIV activity of andrographolide derivatives not seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Cytotoxicity test and cytopathic inhibition test of andrographolide and its derivatives
[0046] I. Materials and methods
[0047] 1. Determination of Drugs and Compounds
[0048] Test samples andrographolide and its derivatives 1-22#. Positive control compound zidovudine
[0049] (3'-Azido-3'-deoxythymidine, AZT) was purchased from Sigma. The sample to be tested was dissolved in DMSO, the concentration of the stock solution was 25mg / ml, and the storage condition was: 4°C; AZT was dissolved in RPMI-1640 complete medium, sterilized by 0.22μm filter membrane, and stored at -20°C after aliquoting.
[0050] 2. Reagents and solutions
[0051] (1) Reagent
[0052] HEPES(N-2(2-Hydroxyethyl)piperazine-N'-(2-ethanesufonic acid), MTT(3,(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), DMF(N , N'-Dimethylformamine), penicillin (Penicillin), streptomycin sulfate (Streptomycin sulfate), glutamine (Glutamine) were all purchased from Sigma Company; 2-mercap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com