Phenylbutyryl curcumin derivate and application thereof in anti-tumor drug preparation
A technology of phenylbutyryl curcumin and anti-tumor drugs, which is applied in the application field of phenylbutyryl curcumin derivatives and their preparation, and preparation of anti-tumor drugs, which can solve the problem of low bioavailability, restriction of curcumin, and difficulty in achieving effective Concentration and other issues, to achieve the effect of increased life extension rate and strong anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0032] The synthesis of embodiment 14-[two (2-chloroethyl) amino] phenylbutyryl curcumin (compound 1)
[0033] EDCI 1.92g (10mmol) was dissolved in anhydrous dichloromethane 200ml, stirred under ice bath and added curcumin 7.36g (20mmol), DMAP 0.244g (2mmol), then slowly added dropwise dissolved 4-[bis(2 -Chloroethyl)amino]benzenebutyric acid 3.04g (10mmol) in dichloromethane 200ml, continued stirring reaction at room temperature for 6 hours. Wash with distilled water, dry the organic phase with anhydrous magnesium sulfate, filter and concentrate to obtain the crude product, the crude product is separated by medium pressure preparative chromatography, reverse C18 column, mobile phase methanol: water 50% → 100% (volume percentage) gradient elution, Obtain 4-[two (2-chloroethyl) amino] phenylbutyryl curcumin pure product 1.30g, productive rate 20%, chemical structural formula is as follows:
[0034]
[0035] Molecular formula C 35 h 37 Cl 2 NO 7 , mp100-102℃
[0036] 1...
Embodiment 24
[0037] Embodiment 24, the synthesis of 4'-[bis(2-chloroethyl)amino]bisphenylbutyryl curcumin (compound 2)
[0038] EDCI 1.92g (10mmol) was dissolved in anhydrous dichloromethane 100ml, stirred under ice bath and added curcumin 1.84g (5mmol), DMAP 0.122g (1mmol), then slowly added dropwise dissolved 4-[bis(2 -Chloroethyl)amino]benzenebutyric acid 3.04g (10mmol) in dichloromethane 200ml, continue stirring reaction at room temperature for 6 hours. Wash with distilled water, dry the organic phase with anhydrous magnesium sulfate, filter and concentrate to obtain the crude product, the crude product is separated by medium pressure preparative chromatography, reverse C18 column, mobile phase methanol: water 50% → 100% (volume percentage) gradient elution, Obtain 4,4'-[bis(2-chloroethyl) amino] bisphenylbutyryl curcumin pure product (compound 2) 1.88g, productive rate 40%, chemical structural formula is as follows:
[0039]
[0040] C 49 h 54 C 14 N 2 o 8 , mp58-60℃, 1 H NM...
Embodiment 3
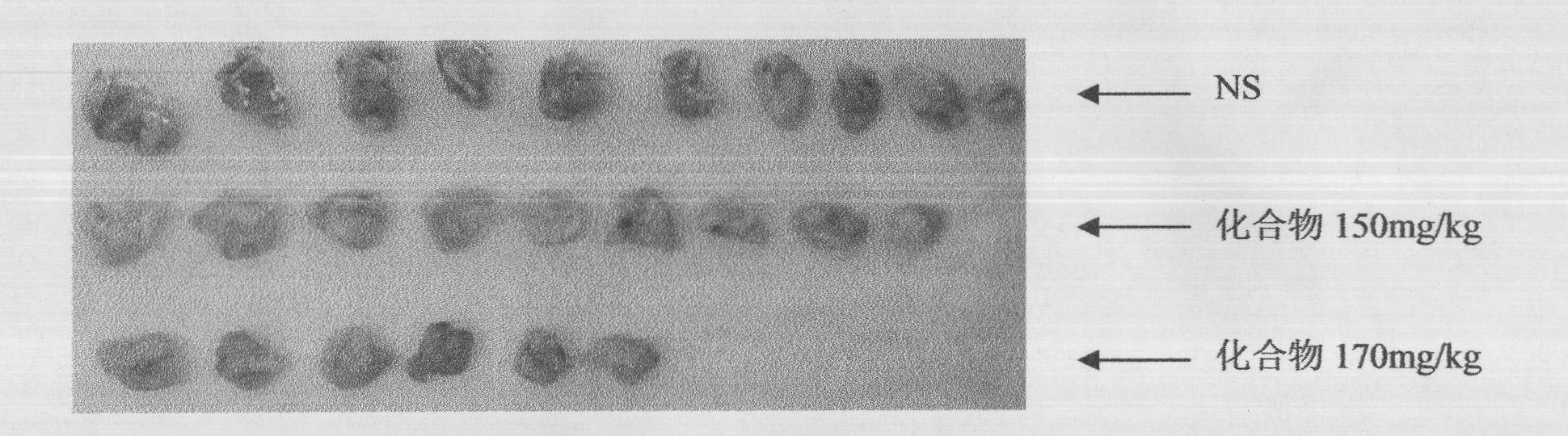
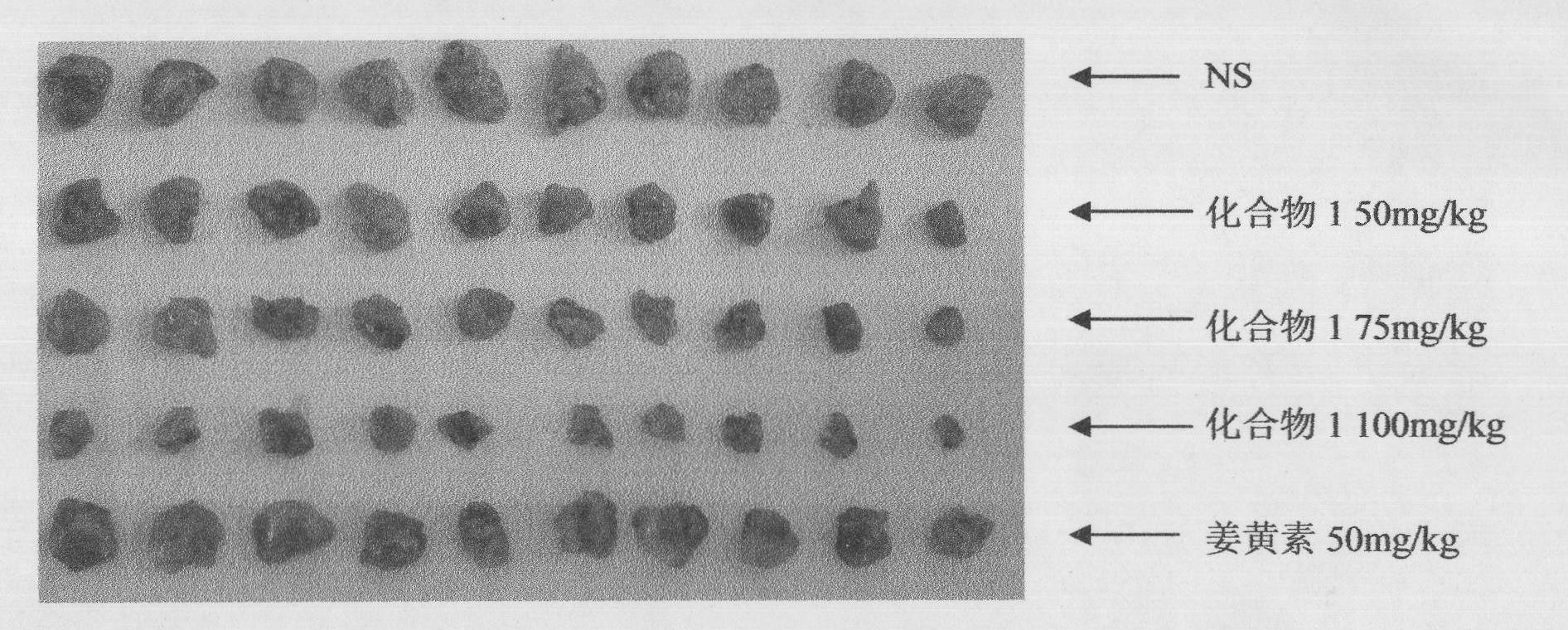
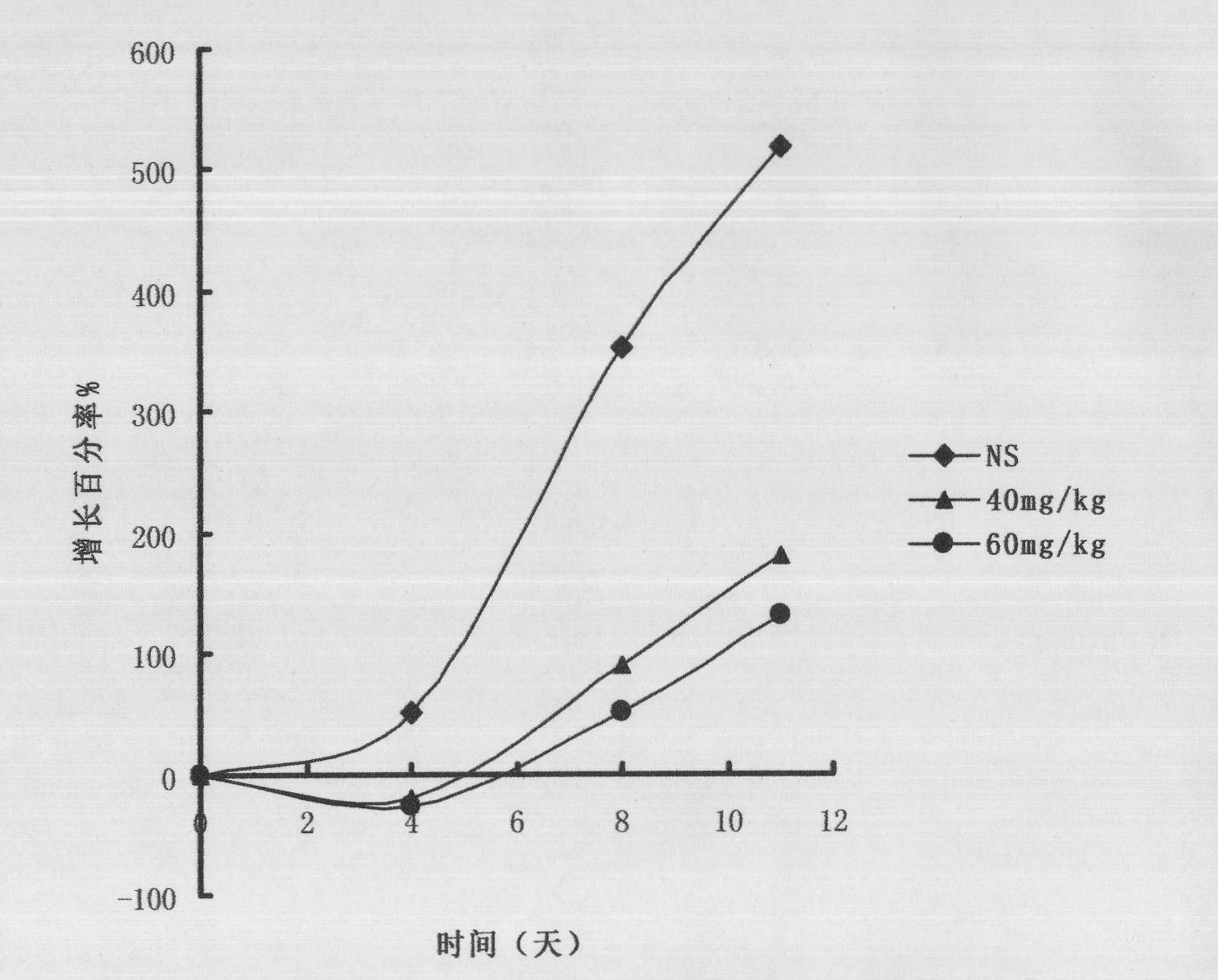
[0041] Example 3 Effect of compound 1 on mouse liver cancer H22 xenograft model
[0042] 3.1 Materials
[0043] 8-12 weeks old Kunming healthy mice, female, weighing (20±2) g; the mice were provided by the Experimental Animal Center of Fujian Medical University (Certificate No. SCXK (Fujian) 200420002). Mouse hepatoma cell line H22. Compound 1 was formulated to the required concentration before use.
[0044] 3.2 Method
[0045] 3.2.1 Establishment of tumor-bearing mouse model
[0046] H22 tumor cells were passed on for more than two generations, and the number of cells was adjusted to 10 7 / ml, 0.2mL / mouse was inoculated subcutaneously in the right forelimb of mice, inoculated 2 mice, waited for about two weeks, stripped the tumor, homogenized, and inoculated 80 mice.
[0047] 3.2.2 Grouping
[0048] A: Divided into 3 groups: the mice 24 hours after the inoculation of tumor strains were randomly divided into 3 groups: Group I was the normal saline control group, compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com