Production method of low-residue tetrabromobisphemol A brominated epoxy resin
A technology of brominated epoxy resin and tetrabromobisphenol, which is applied in the production field of low residual tetrabromobisphenol A brominated epoxy resin, can solve the problem of tetrabromobisphenol A brominated epoxy resin production process, which has not been reported and other issues to achieve the effect of reducing product cost and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
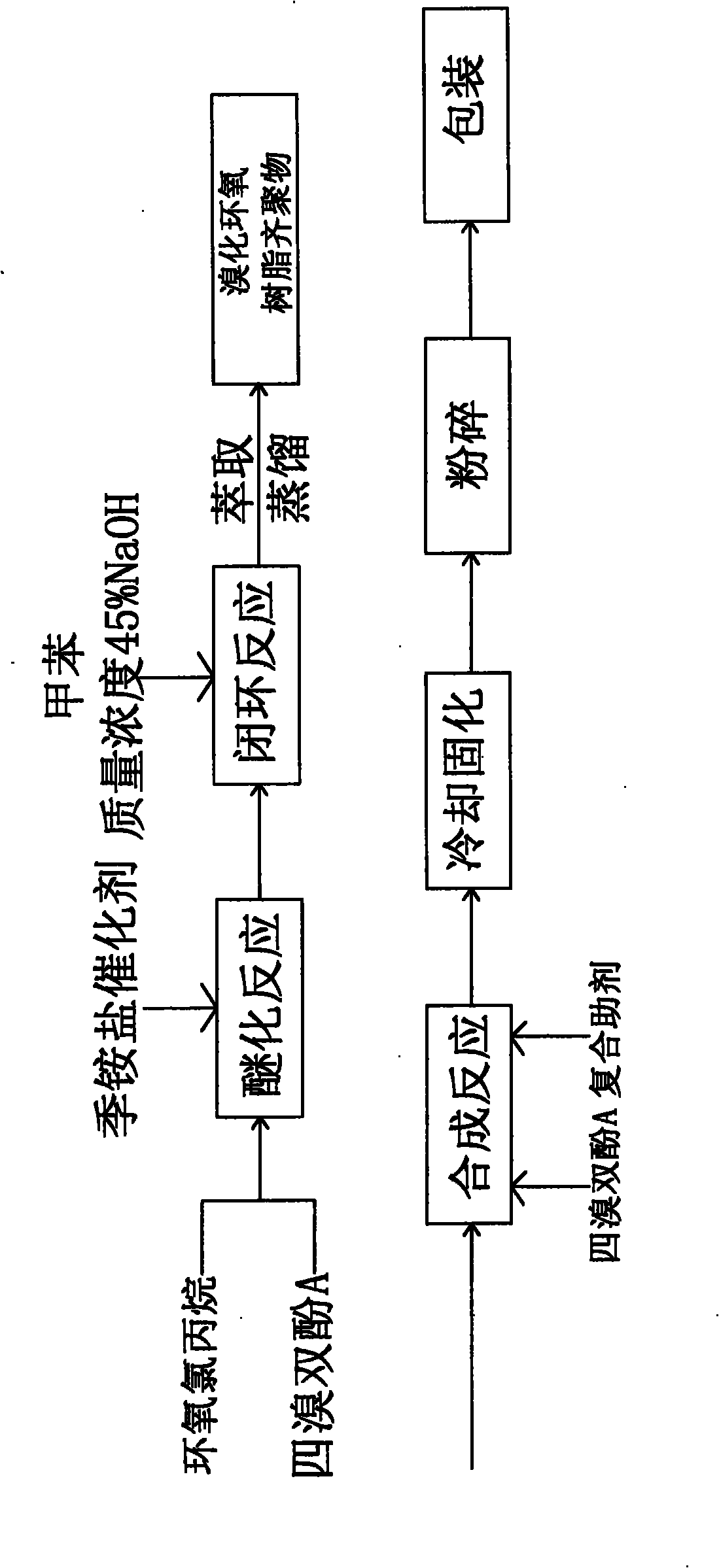
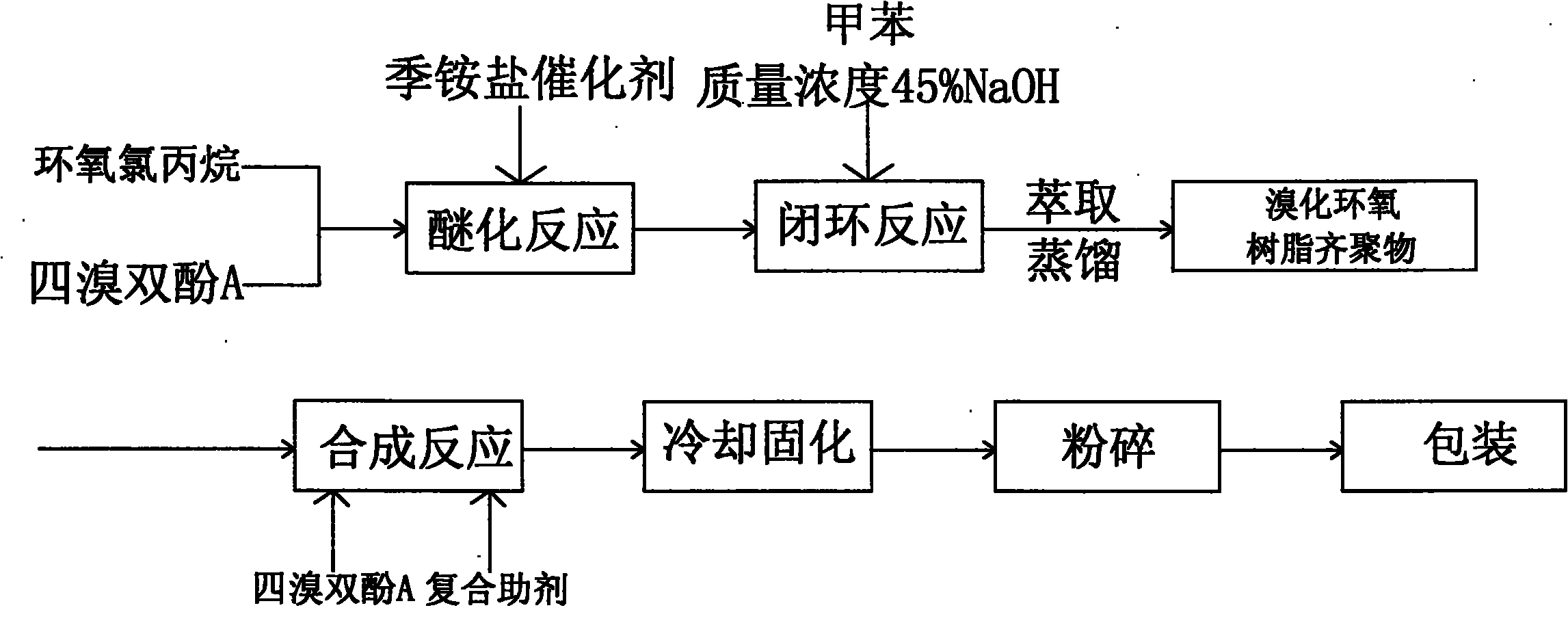
[0020] The production method of low residual tetrabromobisphenol A brominated epoxy resin,
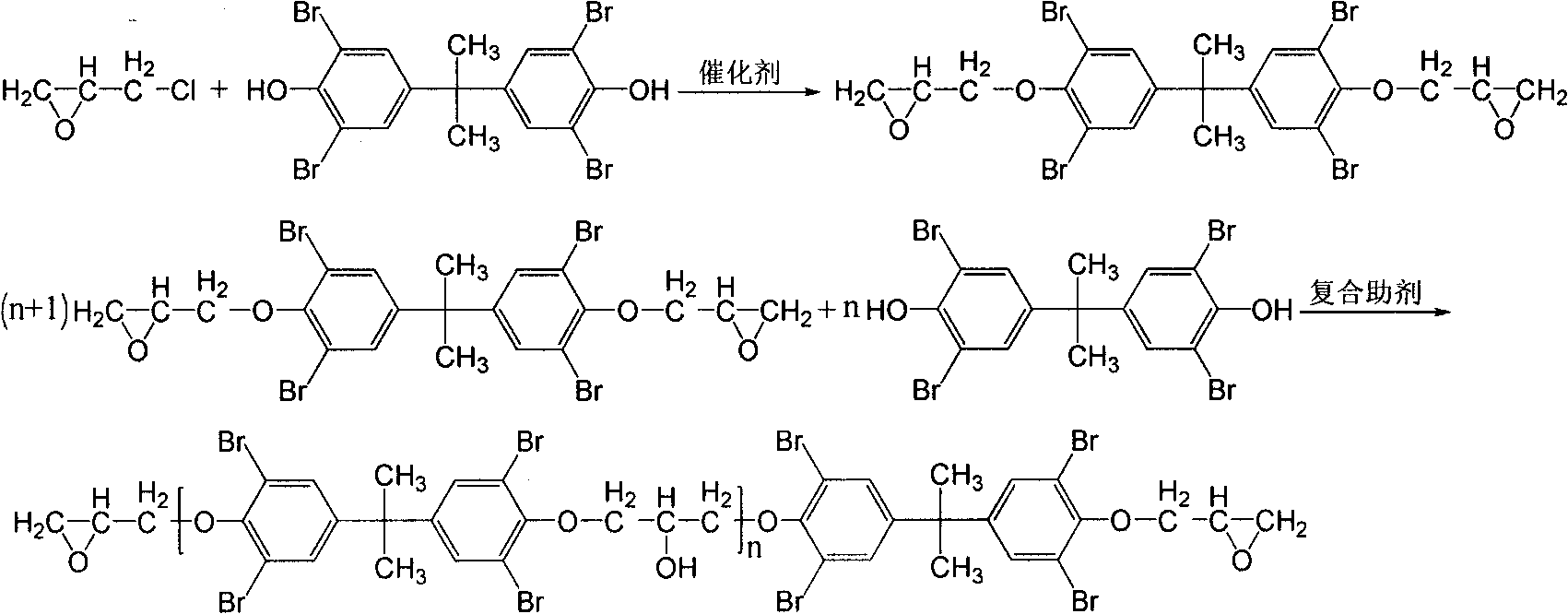
[0021] Step 1, etherification reaction: put 450.3Kg epichlorohydrin and 540.5Kg tetrabromobisphenol A into the etherification reaction kettle, add 4.95Kg catalyst benzyltriphenylammonium bromide, etherification temperature 94°C, etherification reaction 3.5h.
[0022] Step 2, ring-closing reaction: vacuumize to remove excess epichlorohydrin, then put in 150.3Kg toluene, control the temperature at about 70°C and put in 260.5Kg of NaOH with a mass concentration of 45% three times for 6 hours of ring-closing reaction.
[0023] Step 3, obtain the oligomer of brominated epoxy resin: put in 198.7Kg of water, add 300.5Kg of toluene, adjust the pH value to 7-8 with sodium dihydrogen phosphate, extract, distill, and recycle toluene to obtain brominated epoxy resin Oligomer 619.3Kg.
[0024] Step 4, synthesis step: After the brominated epoxy resin oligomer is heated, stirred and melted in the s...
Embodiment 2
[0028] The production method of low residual tetrabromobisphenol A brominated epoxy resin,
[0029] Step 1, etherification reaction: put 512.8Kg epichlorohydrin and 615.4Kg tetrabromobisphenol A into the etherification reaction kettle, add 5.64Kg catalyst benzyltriphenylammonium bromide, etherification temperature 95°C, etherification reaction 3.5h.
[0030] Step 2, ring-closing reaction: Vacuumize to remove excess epichlorohydrin, then add 179.3Kg toluene, control the temperature at about 70°C and add 298.6Kg of NaOH with a mass concentration of 45% in three times for 6 hours of ring-closing reaction.
[0031] Step 3, obtain brominated epoxy resin oligomer: put into 226.3Kg water, add 341.4Kg toluene, adjust the pH value to 7-8 with sodium dihydrogen phosphate, extract, distill, reclaim toluene, obtain brominated epoxy resin Oligomer 705.2Kg.
[0032] Step 4, synthesis step: After the brominated epoxy resin oligomer is heated, stirred and melted in the synthesis kettle, 546...
Embodiment 3
[0036] The production method of low residual tetrabromobisphenol A brominated epoxy resin,
[0037] Step 1, etherification reaction: put 1000.2Kg epichlorohydrin and 1200.3Kg tetrabromobisphenol A into the etherification reaction kettle, add 11.02Kg catalyst benzyltriphenylammonium bromide, etherification temperature 93 ℃, etherification reaction 3.5h.
[0038] Step 2, ring-closing reaction: Vacuumize to remove excess epichlorohydrin, then add 340.3Kg toluene, control the temperature at about 70°C and add 542.7Kg of NaOH with a mass concentration of 45% in three times for 6 hours of ring-closing reaction.
[0039] Step 3, obtain the oligomer of brominated epoxy resin: put in 430.1Kg of water, add 609.2Kg of toluene, adjust the pH value to 7-8 with sodium dihydrogen phosphate, extract, distill, and recover toluene to obtain brominated epoxy resin Oligomer 1380.6Kg.
[0040] Step 4, synthesis step: After the brominated epoxy resin oligomer is heated, stirred and melted in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com