Process and device for purifying sulfuric acid phase in iodine and sulfur cycle under low pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
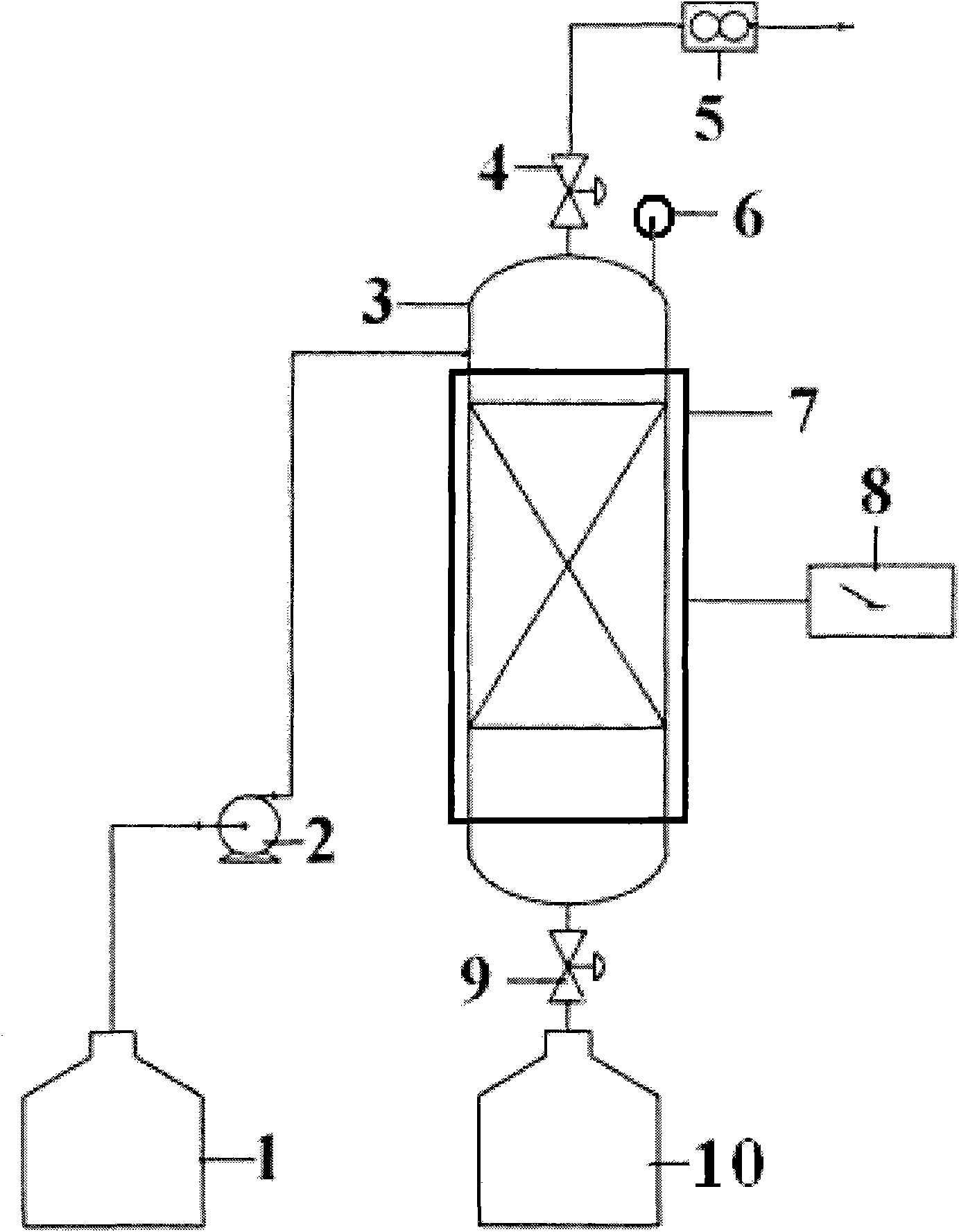
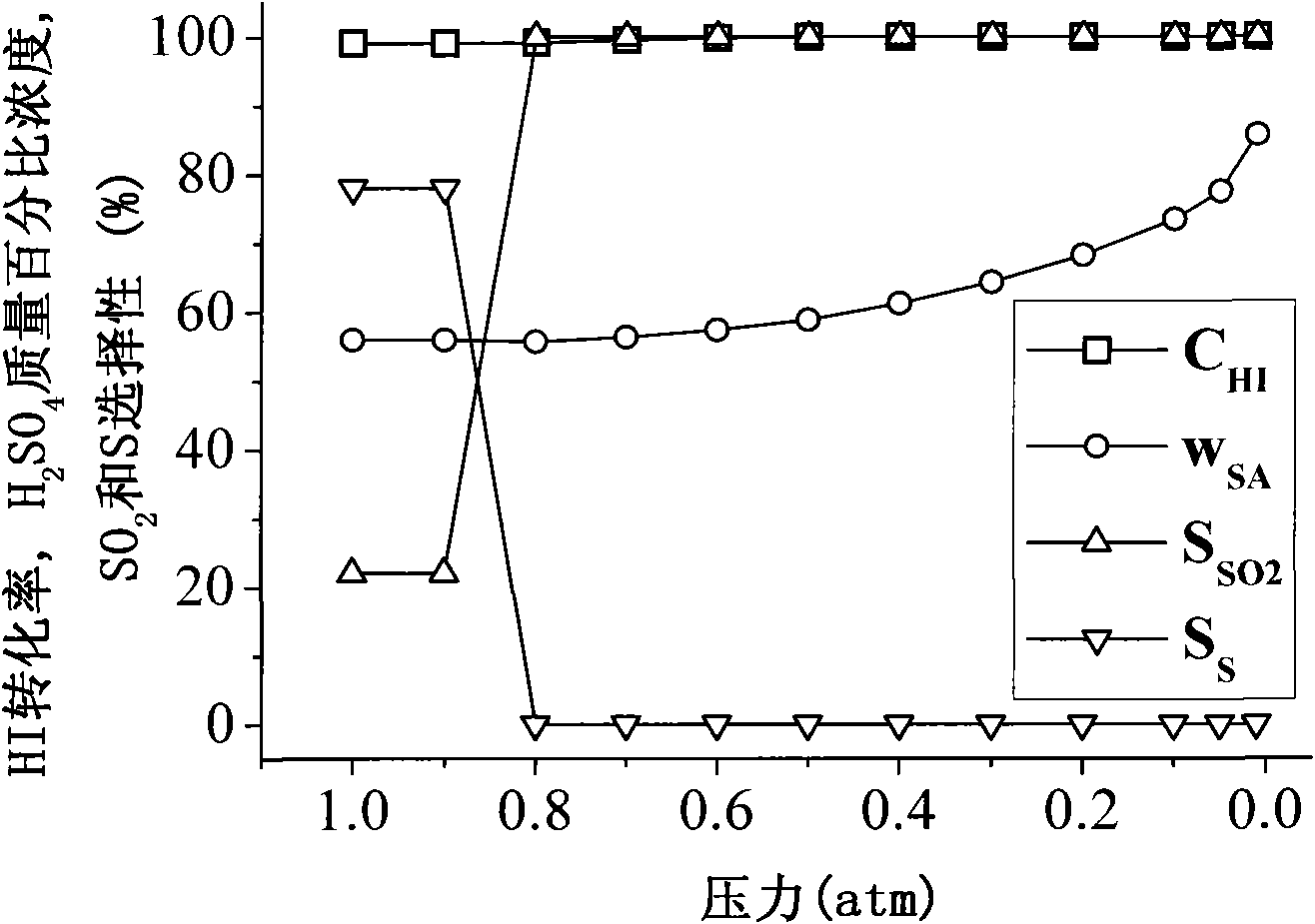
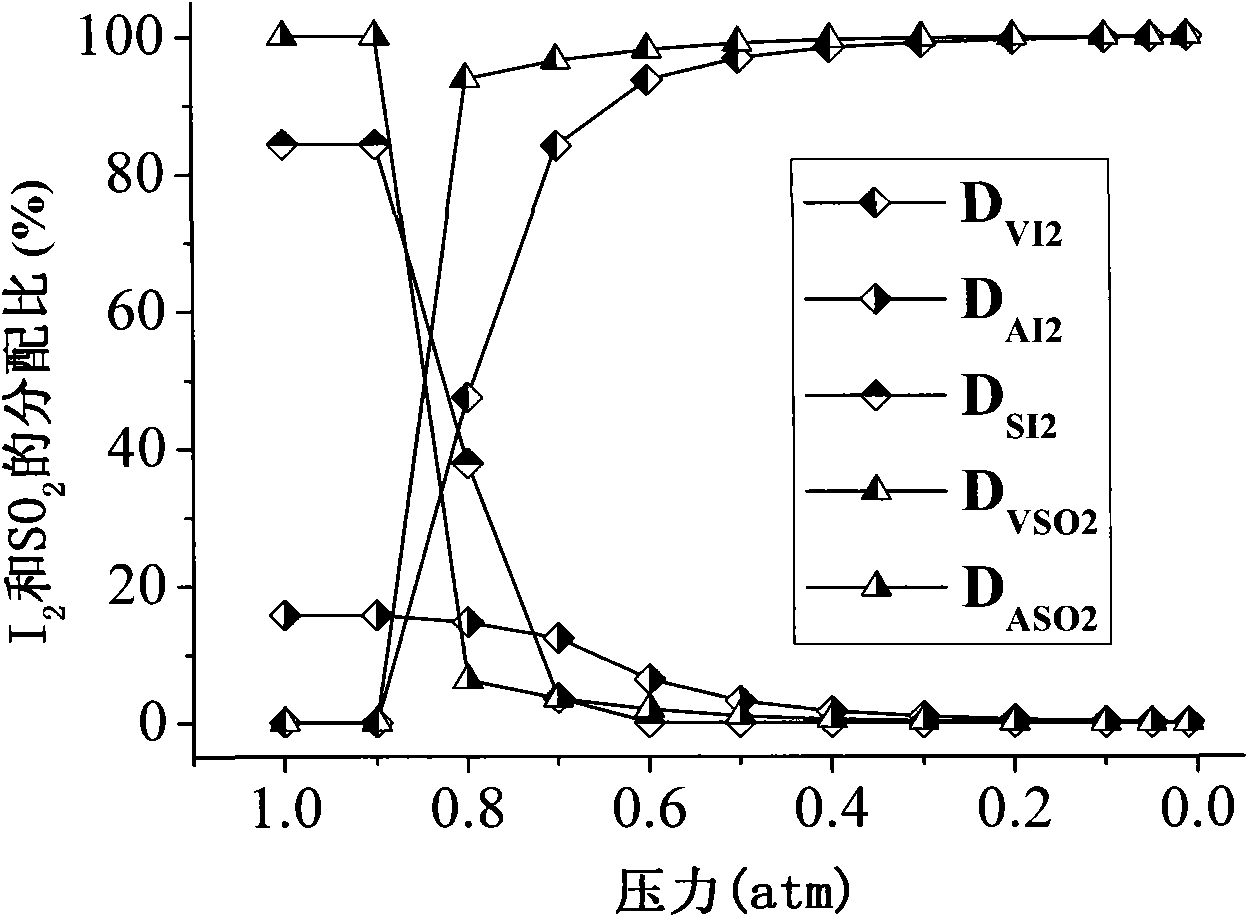
[0033] First, the purification and concentration tower (such as figure 1 Shown) heating up to 110°C, using a vacuum control pump to control the pressure P of the purification and concentration tower to 1atm (as a comparison), 0.9atm, 0.8atm, 0.7atm, 0.6atm, 0.5atm, 0.4atm, 0.3atm, 0.2 atm, 0.1atm, 0.05atm, 0.01atm, through the liquid flow control pump will be composed into H 2 SO 4 +0.1HI+4H 2 O (ie molar ratio H 2 SO 4 :HI:H 2 (0=1: 0.1: 4) sulfuric acid phase, input from the upper feed port of the purification and concentration tower, the liquid flow rate of the control sulfuric acid phase is 183g / h, and the sulfuric acid phase flows through the purification and concentration tower of temperature control temperature and low pressure, a small amount of in the sulfuric acid phase The reverse reaction of Benson's reaction between hydroiodic acid and partial sulfuric acid: H 2 SO 4 +2HI=SO 2 +I 2 +2H 2 O, the SO obtained from the purification reaction 2 , I 2 and H ...
Embodiment 2
[0036] First, the purification and concentration tower (such as figure 1 Shown) heating up to 60°C, using a vacuum control pump to control the pressure P of the purification and concentration tower to 0.1atm, 0.09atm, 0.08atm, 0.07atm, 0.06atm, 0.05atm, 0.04atm, 0.03atm, 0.02atm, 0.01 atm, by liquid flow control pump will be composed into H 2 SO 4 +0.1HI+4H 2 O (ie molar ratio H 2 SO 4 :HI:H 2 (0=1: 0.1: 4) sulfuric acid phase, input from the upper feed port of the purification and concentration tower, the liquid flow rate of the control sulfuric acid phase is 183g / h, and the sulfuric acid phase flows through the purification and concentration tower of temperature control temperature and low pressure, a small amount of in the sulfuric acid phase The reverse reaction of Benson's reaction between hydroiodic acid and partial sulfuric acid: H 2 SO 4 +2HI=SO 2 +I 2 +2H 2 O, the SO obtained from the purification reaction 2 , I 2 and H 2 O is extracted from the exhaust p...
Embodiment 3
[0039] First, the purification and concentration tower (such as figure 1 Shown) heating up to 60°C, 80°C, 100°C, 120°C, 140°C, 160°C, 180°C, 200°C respectively, using the vacuum control pump to control the pressure P of the purification and concentration tower to 0.06atm, and control the The pump will consist of H 2 SO 4 +0.1HI+4H 2 O (ie molar ratio H 2 SO 4 :HI:H 2 (0=1: 0.1: 4) sulfuric acid phase, input from the upper feed port of the purification and concentration tower, the liquid flow rate of the control sulfuric acid phase is 183g / h, and the sulfuric acid phase flows through the purification and concentration tower of temperature control temperature and low pressure, a small amount of in the sulfuric acid phase The reverse reaction of Benson's reaction between hydroiodic acid and partial sulfuric acid: H 2 SO 4 +2HI=SO 2 +I 2 +2H 2 O, the SO obtained from the purification reaction 2 , I 2 and H 2 O is extracted from the exhaust port at the upper end of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com