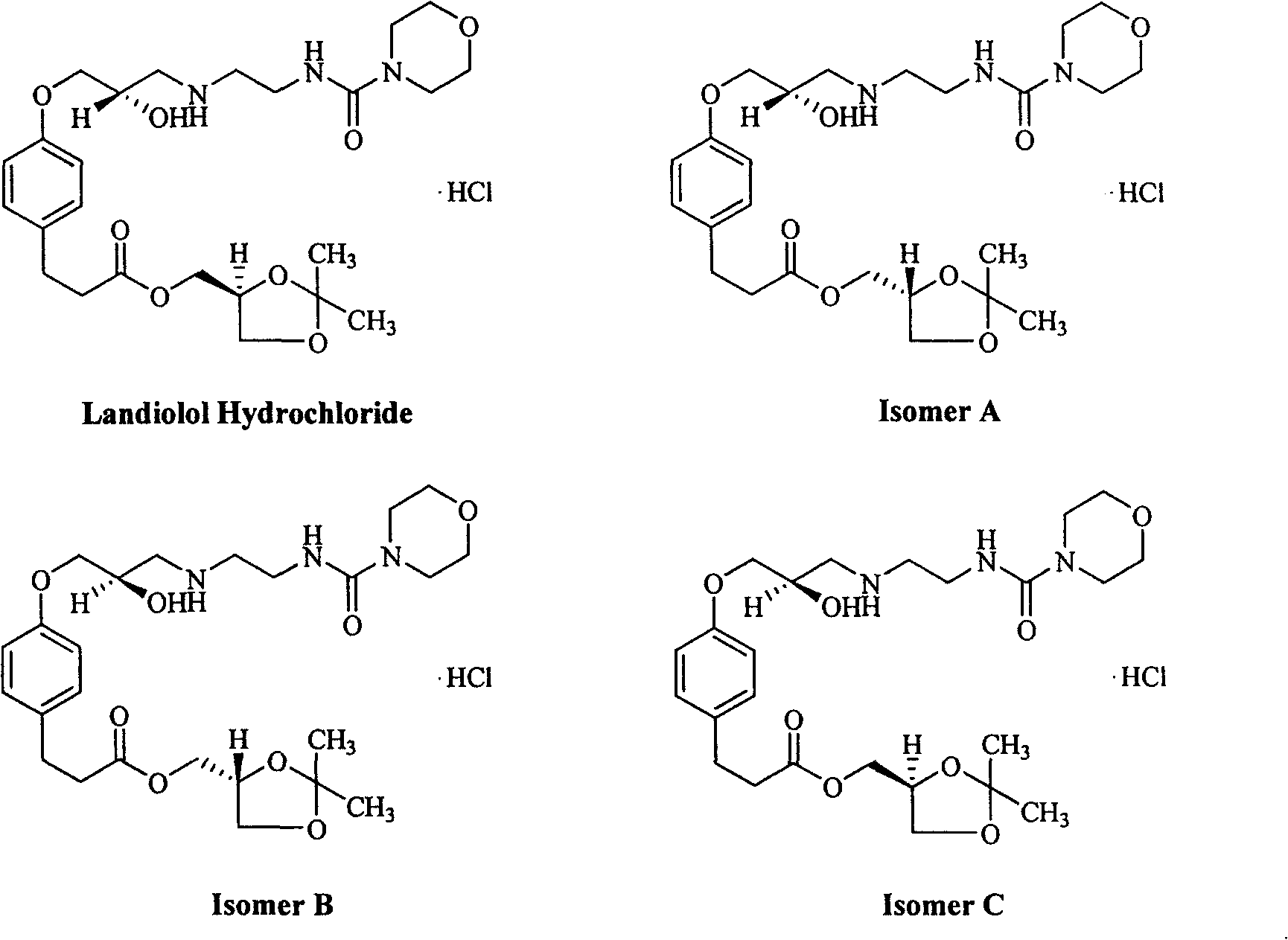
Method for detecting landiolol hydrochloride optical isomers by high efficiency liquid chromatography
A technology of landiolol hydrochloride and high performance liquid chromatography, which is applied in the field of detecting the optical isomers of landiolol hydrochloride, can solve the problems of unsuitable target detection substance, influence of detection results and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
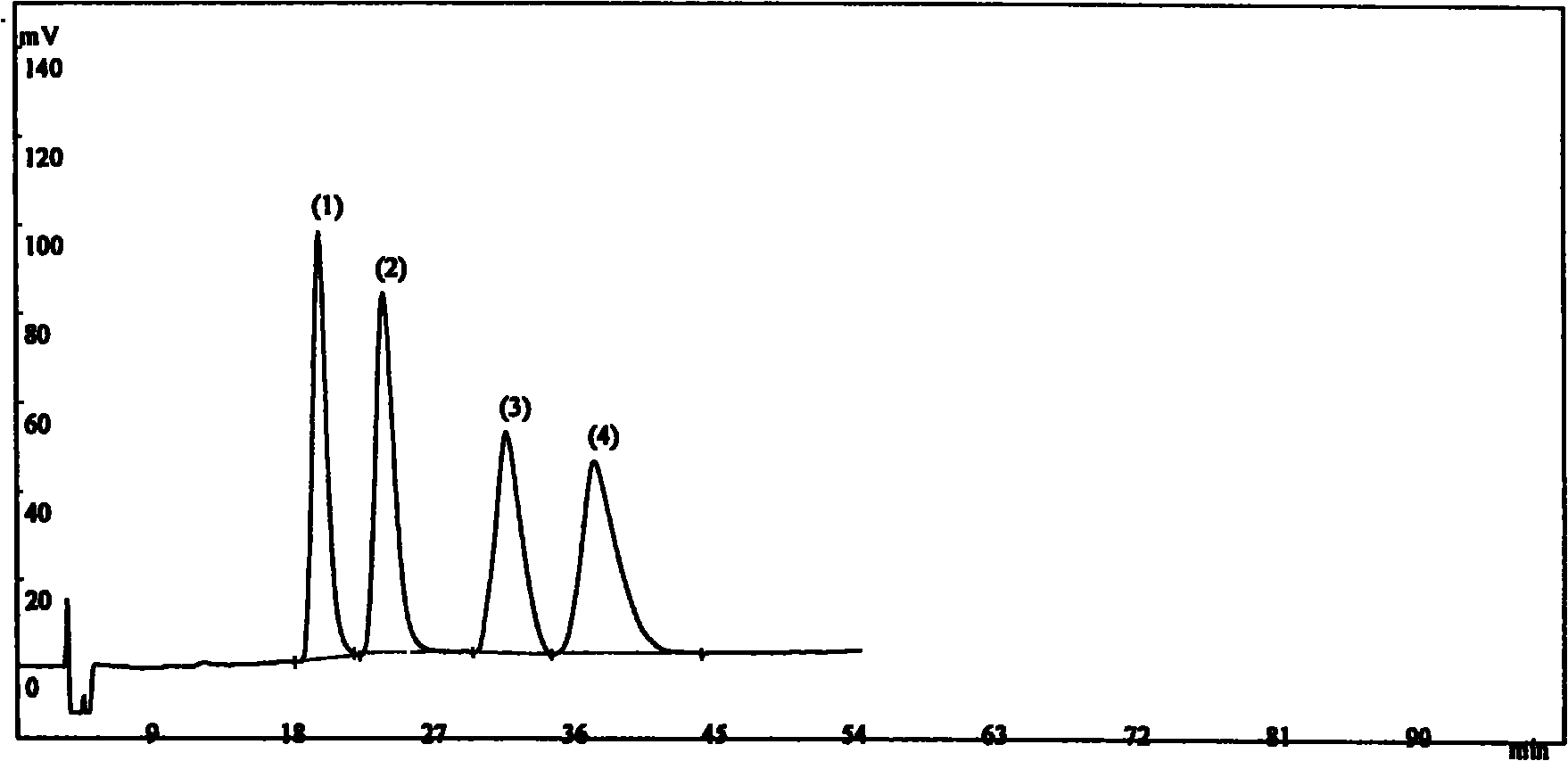
[0016] Instrument: SHIMADZU LC-10AT high performance liquid chromatograph, SPD-10Avp ultraviolet detector, OV-100 column thermostat;
[0017] Chromatographic column: Daicel Chralcel OJ-H chromatographic column (0.46×25cm, 5μm);
[0018] Mobile phase: n-hexane-isopropanol-diethylamine (80:20:0.1)
[0019] Column temperature: 35°C
[0020] Flow rate: 1.0ml / min
[0021] Detection wavelength: 220nm.
[0022] Preparation of Positioning Solution 1: Accurately weigh an appropriate amount of isomer A of Landil Hydrochloride, dissolve and dilute to 2 mg / ml with isopropanol-methanol (50:50);
[0023] Preparation of positioning solution 2: Accurately weigh an appropriate amount of isomer B of Landil hydrochloride, dissolve and dilute to 2 mg / ml with isopropanol-methanol (50:50);
[0024] Preparation of positioning solution 3: Accurately weigh an appropriate amount of isomer C of Landil hydrochloride, dissolve and dilute to 2 mg / ml with isopropanol-methanol (50:50);
[0025] Preparat...
Embodiment 2
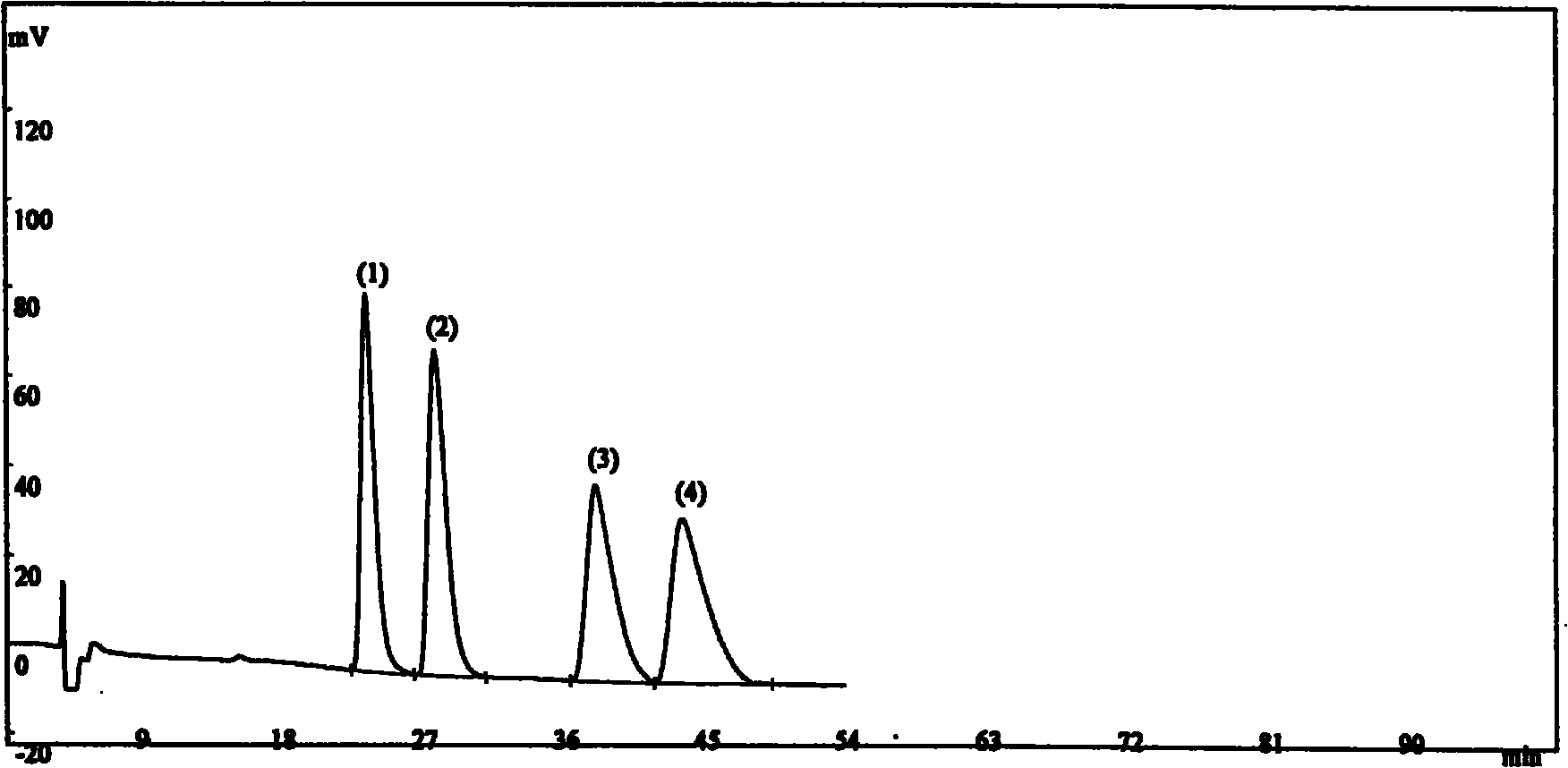
[0028] Instrument: SHIMADZU LC-10AT high performance liquid chromatograph, SPD-10Avp ultraviolet detector, OV-100 column thermostat;
[0029] Chromatographic column: AE.Lichrom OJ-H chromatographic column (0.46×25cm, 5μm);
[0030] Mobile phase: n-hexane-isopropanol-diethylamine (82:18:0.1)
[0031] Column temperature: 35°C
[0032] Flow rate: 1.0ml / min
[0033] Detection wavelength: 220nm.
[0034] Preparation of Positioning Solution 1: Accurately weigh an appropriate amount of isomer A of Landil Hydrochloride, dissolve and dilute to 2 mg / ml with isopropanol-methanol (50:50);
[0035] Preparation of positioning solution 2: Accurately weigh an appropriate amount of isomer B of Landil hydrochloride, dissolve and dilute to 2 mg / ml with isopropanol-methanol (50:50);
[0036] Preparation of positioning solution 3: Accurately weigh an appropriate amount of isomer C of Landil hydrochloride, dissolve and dilute to 2 mg / ml with isopropanol-methanol (50:50);
[0037] Preparation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com